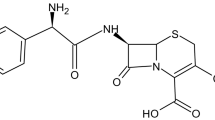
Abstract
A revision on articles concerning thermal analysis of tetracyclines is presented, regarding drug-excipient compatibility, characterization of drug releasing systems, physicochemical behavior of drug-supported systems and degradation of some pharmaceuticals itself. Initially, an overview of information regarding this class of drugs is presented. A bibliographic survey revealed relatively few works on this subject despite the worldwide use and relevance of this class of antibiotics. Here, 37 papers published during the time frame 2000–2022 were classified as above and discussed once they presented thermoanalytical data straightly related to the tetracyclines. From the thermal studies of the drugs, a general decomposition behavior could be identified and it is presented.


Adapted from Web of Science


Similar content being viewed by others
Abbreviations
- TCs:
-
General abbreviation for tetracyclines
- TC:
-
Tetracycline
- TCH:
-
Tetracycline hydrochloride
- OTC:
-
Oxytetracycline
- DOX:
-
Doxycycline
- DOX-SLN:
-
Doxycycline-loaded solid lips nanoparticles
- OTCH:
-
Oxytetracycline hydrochloride
- CTCH:
-
Chlortetracycline hydrochloride
- MNC:
-
Minocycline
- AHTC:
-
Anhydrotetracycline
- Meclo:
-
Meclocycline
- TGA:
-
Thermogravimetry
- DTG:
-
Derivative thermogravimetry
- DTA:
-
Differential thermal analysis
- DSC:
-
Differential scanning calorimetry
- EGA:
-
Evolved gas analysis
- TGA-FTIR:
-
Thermogravimetry coupled to Fourier transform infrared spectroscopy
- AFM:
-
Atomic force microscopy
- DLS:
-
Dynamic light scattering
- FTIR:
-
Fourier transform infrared spectroscopy
- 31P-NMR :
-
Phosphorus-31 nuclear magnetic resonance
- 1H-NMR :
-
Hydrogen nuclear magnetic resonance
- XRPD:
-
X-ray powder diffraction
- VLI:
-
Visible light irradiation
- Rp:
-
Propagation rate constant
- IP:
-
Induction period
- AIBN:
-
Azobisisobutyronitrile
- BPO:
-
Benzoyl peroxide
- BSA:
-
Bovine serum albumin
- CTAB:
-
Cetrimonium bromide
- HCECs:
-
Human corneal eukaryotic cells
- HPMC:
-
Hydroxypropyl methylcellulose
- k-carrageenan:
-
Kappa carrageenan
- MMA:
-
Methyl methacrylate
- MMW:
-
Miracle mouthwash
- Mt:
-
Montmorillonite
- Pal:
-
Palygorskite
- PCL:
-
poly(ε-Caprolactone)
- PLA-HAP-DOX:
-
Polylactic acid-hydroxyapatite-doxycycline
- PLGA:
-
poly(D,L-Lactide-co-glycolide)
- PLLA:
-
poly(L-lactic acid)
- PLLA-PEG-PLLA:
-
poly(L,L-Lactide)-b-poly(ethylene glycol)-b-poly(L,L-lactide)
- PMMA:
-
poly(Methyl methacrylate)
- PVA-SbQ:
-
poly(Vinyl alcohol)-stilbazole quaternized
- RAL:
-
Raloxifene HCl
- Sep:
-
Sepiolite
- SLST:
-
Surfactants sodium lauryl sulfate
- Veegum© :
-
Montmorillonite
References
Lindblad WJ. Considerations for determining if a natural product is an effectivewound-healing agent. Int J Low Extrem Wounds. 2008;7:75–81.
Forrest RD. Early history of wound treatment. J R Soc Med. 1982;75:198–205.
Wainwright M. Moulds in ancient and more recent medicine. Mycologist. 1989;3:21–3.
https://www.nobelprize.org/prizes/medicine/1908/summary/; accessed in: December 2022
Chambers HF. General considerations of antimicrobial therapy. Goodman & Gilman's The Pharmacological Basis of Therapeutics. LL Brunton; JS Lazo, KL Parker eds., 3rd ed. McGraw Hill:New York; 2006.
Finberg RW, Moellering RC, Tally FP, Craig WA, Pankey GA, Dellinger EP. The importance of bactericidal drugs: future directions in infectious disease. Clin Infect Dis. 2004;39:1314–20.
Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65:232–60.
IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. ISBN 0–9678550–9–8. https://doi.org/10.1351/goldbook.
O’Neil MJ, Heckelman PE, Dobbelaar PH. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biological. 15th ed. Cambridge - UK: Royal Society of Chemistry; 2013.
Granados-Chinchilla F, Rodriguez C. Tetracyclines in food and feeding stuffs: from regulation to analytical methods, bacterial resistance, and environmental and health implications. J Anal Methods Chem. 2017;2017:1315497.
Deboyser D, Goethals F, Krack G, Roberfroid M. Investigation into the mechanism of tetracycline-induced steatosis: study in isolated hepatocytes. Toxicol Appl Pharmacol. 1989;97:473–9.
Amacher DE, Martin BA. Tetracycline-induced steatosis in primary canine hepatocyte cultures. Fundam Appl Toxicol. 1997;40:256–63.
Ekwall B, Acosta D. In vitro comparative toxicity of selected drugs and chemicals in HeLa cells, Chang liver cells, and rat hepatocytes. Drug Chem Toxicol. 1982;5:219–31.
Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: a myth debunked. J Am Acad Dermatol. 2022;46:917–23.
Dréno B, Bettoli V, Ochsendorf F, Layton A, Mobacken H, Degreef H. European recommendations on the use of oral antibiotics for acne. J Eur Acad Dermatol. 2004;14(6):391–9.
DeRossi SS, Hersh EV. Antibiotics and oral contraceptives. J Eur Acad Dermatol. 2002;46:653–64.
Mother to Baby | Fact Sheets [Internet]. Brentwood (TN): Organization of Teratology Information Specialists (OTIS); 1994. Tetracycline. 2022 Feb. PMID: 35952247.
Clarindo JES, Viana RB, Cervini P, Silva ABF, Cavalheiro ETG. Determination of tetracycline using a graphite-polyurethane composite electrode modified with a molecularly imprinted polymer. Anal Lett. 2020;53:1932–55.
Calixto CMF, Cavalheiro ETG. Determination of tetracycline in bovine and breast milk using a graphite-polyurethane composite electrode. Anal Lett. 2017;50:2323–34.
Calixto CMF, Cervini P, Cavalheiro ETG. Determination of tetracycline in environmental water samples at a graphite-polyurethane composite electrode. J Braz Chem Soc. 2012;23:938–43.
Bassett EJ, Keith MS, Armelagos GJ, Martin DL, Villanueva AR. Tetracycline-labeled bone from ancient Sudanese Nubia (A.D. 350). Science. 1980;9:1532–4.
Samuel D. Archaeology of ancient Egypt beer. J Am Soc Brew Chem. 1996;54:3–12.
Nelson ML, Dinardo A, Hochberg J, Armelagos GJ. Mass spectroscopic characterization of tetracycline in the skeletal remains of an ancient population from Sudanese Nubia 350–550 CE. Am J Phys Anthropol. 2010;143:151–4.
Borgui AA, Palma MSA. Tetracycline: production, waste treatment and environmental impact assessment. Braz J Pharm Sci. 2014;50:25–40.
Craig DQM. Thermal Analysis of Pharmaceuticals. 1st ed. Boca Raton: CRC Press; 2006.
Saunders M. Thermal analysis of pharmaceuticals. In thermal analysis principles and applications. 1st ed. Oxford: Blackwell Pub, Ames, Iowa, 2008.
Giron D. Applications of thermal analysis and coupled techniques in pharmaceuticalindustry. J Therm Anal Calorim. 2002;68:335–57.
https://www.webofscience.com/wos/woscc/basic-search; accessed in: May 2022
Bueno MS, Miñambres GG, Bongioannia A, Chattah AK, Aiassa V, Longhia MR, Garnero C. Exploring solid forms of oxytetracycline hydrochloride. Int J Pharm. 2020;585: 119496.
Tekade R. Dosage form design parameters. 1st ed. Cambridge: Academic Press; 2018.
Narkhede R, Athawale R, Patil N, Baburaj M. Formulation, evaluation, and clinical assessment of novel solid lipid microparticles of tetracycline hydrochloride for the treatment of periodontitis. AAPS Pharm Sci Tech. 2021;22:162–72.
Narkhede RG, Athawale RB. Screening of Selective C16 to C18 Lipids and process optimization based on design of experiments in formulating solid lipid microparticles by twin screw hot melt dispersion process. J Pharm Innov. 2021;17:940–54.
Patlolla VGR, Popovic N, Peter Holbrook W, Kristmundsdottir T, Gizurarson S. Effect of doxycycline microencapsulation on buccal films: stability. Mucoadhesion and in vitro drug release. Gels. 2021;7:51–64.
Younis US, Fazel M, Myrdal PB. Characterization of tetracycline hydrochloride compounded in a miracle mouthwash formulation. AAPS Pharm Sci Tech. 2019;20:178–85.
Johnson ML, Uhrich KE. Concurrent release of admixed antimicrobials and salicylic acid from salicylate-based poly(anhydride-esters). J Biomed Mater Res A. 2009;91A:671–8.
Meretoudi A, Banti CN, Siafarika P, Kalampounias AG, Hadjikakou SK. Tetracycline water soluble formulations with enhanced antimicrobial activity. Antibiotics. 2020;9:845.
Pathak M, Coombes AGA, Turner MS, Palmer C, Wang D, Steadman KJ. Investigation of polycaprolactone matrices for intravaginal delivery of doxycycline. J Pharm Sci. 2015;104:4217–22.
Misra R, Acharya S, Dilnawaz F, Sahoo SK. Sustained antibacterial activity of doxycycline-loaded poly(D, L-lactide-co-glycolide) and poly(ε-caprolactone) nanoparticles. Nanomedicine. 2009;4:519–30.
Turan CU, Metin A, Guvenilir Y. Controlled release of tetracycline hydrochloride from poly(ω-pentadecalactone-co-ε-caprolactone)/gelatin nanofibers. Eur J Pharm Biopharm. 2021;162:59–69.
Cui J, Wang Q-Q, Qiu Y-Y, Wei Q-F. Electrospun poly(vinyl alcohol)-stilbazole quaternized/zein-tetracycline hydrochloride core-sheath nanofibers for drug release. J Nanosci Nanotechnol. 2016;16:9497–504.
Gill P, Moghadam TT, Ranjbar B. Differential scanning calorimetry techniques: applications in biology and nanoscience. J Biomol Tech. 2010;21:167–93.
Keswani N, Choudhary S, Kishore N. Quantitative aspects of recognition of the antibiotic drug oxytetracycline by bovine serum albumin: calorimetric and spectroscopic studies. J Chem Thermodyn. 2013;58:196–205.
Burgos MI, Fernández RA, Celej MS, Rossi LI, Fidelio GD, Dassie SA. Binding of the highly toxic tetracycline derivative, anhydrotetracycline, to bovine serum albumin. Biol Pharm Bull. 2011;34:1301–6.
Malekar SA, Sarode AL, Bach AC, Bose A, Bothun G, Worthen DR. Radio frequency-activated nanoliposomes for controlled combination drug delivery. AAPS Pharm Sci Technol. 2015;16:1335–43.
Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin Drug Deliv. 2010;7:429–44.
Langer R. New methods of drug delivery. Science. 1990;249:1527–33.
Goh CF, Lane ME. Advanced structural characterisation of pharmaceuticals using nano-thermal analysis (nano-TA). Adv Adv Drug Deliv Rev. 2022;180: 114077.
Li C-Y, Qiu Y, Song D-D. Application of thermal analysis in new drug delivery systems and products. Chinese J New Drugs. 2015;24:654–8.
Agostinho DAS, Paninho AI, Cordeiro T, Nunes AVM, Fonseca IM, Pereira C. Properties of κ-carrageenan aerogels prepared by using different dissolution media and its application as drug delivery systems. Mater Chem Phys. 2020;253: 123290.
Kirchberg M, Eick S, Buchholz M, Rosche F, Kiesow A, Sarembe S. Controlled release minocycline-lipid-complex extrudates for the therapy of periodontitis with enhanced flexibility. Int J Pharm. 2020;586: 119578.
Prasad LTS, Madhusudhan B, Kodihalli BP, Ghosh PC. Development and in vitro evaluation of oxytetracycline loaded PMMA nanoparticles for oral delivery against anaplasmosis. IET Nanobiotechnol. 2017;11:119–26.
Adi H, Young PM, Chan H-K, Stewart P, Agus H, Traini D. Cospray dried antibiotics for dry powder lung delivery. J Pharm Sci. 2008;97:3356–66.
Honary S, Zahir F. Effect of process factors on the properties of doxycycline nanovesicles. Trop J Pharm Res. 2012;11:169–75.
Marques MS, Zepon KM, Petronilho FC, Soldi V, Kanis LA. Characterization of membranes based on cellulose acetate butyrate/poly(caprolactone)triol/doxycycline and their potential for guided bone regeneration application. Mater Sci Eng C. 2017;76:365–73.
Hosseini SM, Abbasalipourkabir R, Jalilian FA, Asl SS, Farmany A, Roshanaei G. Doxycycline-encapsulated solid lipid nanoparticles as promising tool against brucella melitensis enclosed in macrophage: a pharmacodynamics study on J774A1 cell line. Antimicrob Resist Infect Control. 2019;862:1–12.
He C, Huang Z, Han X, Liu L, Zhang H, Chen L. Coaxial Electrospun poly(L-lactic acid) ultrafine fibers for sustained drug delivery. J Macromol Sci B. 2006;45:515–24.
Farkas N-I, Marincaș L, Barabás R, Bizo L, Ilea A, Turdean GL. Preparation and characterization of doxycycline-loaded electrospun PLA/HAP nanofibers as a drug delivery system. Materials. 2022;15:1–16.
Mundargi RC, Srirangarajan S, Agnihotri SA, Patil SA, Ravindra S, Setty SB. Development and evaluation of novel biodegradable microspheres based on poly(d, l-lactide-co-glycolide) and poly(ε-caprolactone) for controlled delivery of doxycycline in the treatment of human periodontal pocket: in vitro and in vivo studies. J Control Release. 2007;119:59–68.
Patel RS, Cho DY, Tian C, Chang A, Estrellas KM, Lavin D. Doxycycline delivery from PLGA microspheres prepared by a modified solvent removal method. J Microencapsul. 2012;29:344–52.
He C-L, Huang Z-M, Han X-J. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. J Biomed Mater Res A. 2009;89A:80–95.
Ghadiri M, Hau H, Chrzanowski W, Agus H, Rohanizadeh R. Laponite clay as a carrier for in situ delivery of tetracycline. RSC Adv. 2013;3:20193.
Andrade ÂL, Militani IA, de Almeida KJ, Belchior JC, dos Reis SC, Costa e Silva RMF. Theoretical and experimental studies of the controlled release of tetracycline incorporated into bioactive glasses. AAPS Pharm Sci Tech. 2018;19:1287–96.
Merchan M, Sedlarikova J, Friedrich M, Sedlarik V, Saha P. Thermoplastic modification of medical grade polyvinyl chloride with various antibiotics: effect of antibiotic chemical structure on mechanical, antibacterial properties, and release activity. Polym Bull. 2011;67:997–1016.
Liebenberg W, de Villiers MM, Wurster DE, Swanepoel E, Dekker TG, Lötter AP. The effect of polymorphism on powder compaction and dissolution properties of chemically equivalent oxytetracycline hydrochloride powders. Drug Dev Ind Pharm. 1999;25:1027–33.
Lu H, Qiu Y, Wang Q, Li G, Wei Q. Nanocomposites prepared by electrohydrodynamics and their drug release properties. Mater Sci Eng C. 2018;91:26–35.
Mothé CG, Azevedo AD, Drumond WS, Wang SH, Sinisterra RD. Preparation and characterization of poly(l, l-lactide)-b-poly(ethylene glycol)-b-poly(l, l-lactide) (PLLA-PEG-PLLA) microspheres having encapsulated tetracycline. J Therm Anal Calorim. 2011;106:671–7.
Rocha MC, Braz EMA, Honório LMC, Trigueiro P, Fonseca MG, Silva-Filho EC. Understanding the effect of UV light in systems containing clay minerals and tetracycline. Appl Clay Sci. 2019;183: 105311.
Yildiz US. Differential scanning calorimetry as a tool to detect antibiotic residues in ultra high temperature whole milk. Int J Food Sci Technol. 2009;44:2577–82.
Murakami Y, Kawata A, Suzuki S, Fujisawa S. Radical-scavenging and pro-/anti-inflammatory activity of tetracycline and related phenolic compounds with or without visible light irradiation. In vivo. 2020;34:81–94.
Dev RK, Mishra P, Kumar Chaudhary N, Bhattarai A. Synthesis, characterization, and antibacterial evaluation of heteroleptic oxytetracycline-salicylaldehyde complexes. J Chem. 2020;2020:1–10.
Nicolet-ThermoScientifc Co. Nicolet EPA Vapor Phase data-base. Omnic 8.0 software. Madison: ThermoScientifc.
Cervini P, Machado LCM, Ferreira APG, Ambrozini B, Cavalheiro ÉTG. Thermal decomposition of tetracycline and chlortetracycline. J Anal Appl Pyrolysis. 2016;118:317–24.
Cervini P, Ambrozini B, Machado LCM, Ferreira APG, Cavalheiro ÉTG. Thermal behavior and decomposition of oxytetracycline hydrochloride. J Therm Anal Calorim. 2015;121:347–52.
Freitas J, Ferreira APG, Cavalheiro ÉTG. Investigating the thermal behavior of doxycycline and meclocycline. J Therm Anal Calorim. 2022;147:13413–23.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo,2019/22217-8,Eder Cavalheiro,Coordenação de Aperfeiçoamento de Pessoal de Nível Superior,Conselho Nacional de Desenvolvimento Científico e Tecnológico
Author information
Authors and Affiliations
Contributions
JdF involved in bibliographic revision, article selection, writing and revision and editing; APGF involved in bibliographic revision, article selection, writing and revision and editing; ÉTGC involved in conceptualization, article selection, revision and editing, project administration and funding acquisition.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Freitas, J., Ferreira, A.P.G. & Cavalheiro, É.T.G. Thermal analysis of tetracyclines: a review. J Therm Anal Calorim (2023). https://doi.org/10.1007/s10973-023-12308-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-023-12308-9