Abstract
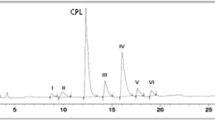
Different forced degradation conditions were tested to evaluate the degradation profile of cephalexin (CEF). Samples of the active pharmaceutical ingredient and the corresponding finished product in capsule form were exposed to stress conditions, namely acid hydrolysis (1 M HCl), basic hydrolysis (0.04 M NaOH), oxidation (H2O2 0.3% and the presence of metal ions (50 mM FeCl3)), thermal treatment at 60 °C, photolytic treatment (2.4 klux h and 400 W h/m2) and controlled humidity (25 °C and 75% relative humidity). The generation of potential degradation products was evaluated. This study allowed for the development of a stability-indicating method using ultrahigh-pressure liquid chromatography coupled with diode array detection and high-resolution mass spectrometry techniques, as well as providing information about possible degradation routes and detection of unknown compounds. The results obtained under forced degradation conditions showed that CEF is generally stable when exposed to temperatures up to 60 °C, ultraviolet light or visible light in the 300–800 nm wavelength range and 75% relative humidity. However, under acidic, basic, hydrogen peroxide and metal ion oxidative conditions, CEF showed degradation susceptibility, as demonstrated by the decrease in content and the formation of degradation products. The results obtained during forced degradation highlight the need for careful evaluation during studies of accelerated stability and the long-term duration of the product, with emphasis on the formation of a degradation product that was observed under all applied stress conditions. This study indicates that caution should be taken regarding hydrolysis of the active pharmaceutical ingredient (API).












Similar content being viewed by others
References
El-Shaboury SR, Saleh GA, Mohamed FA, Rageh AH (2007) Analysis of Cephalosporin antibiotics. J Pharm Biomed Anal 45:1–19
U.S. Food and Drug Administration. Drug Safety and Availability. https://www.fda.gov/drugs/drug-safety-and-availability/drug-recalls. Accessed Dec 2019.
International Conference on Harmonisation (2006) Impurities in New Drug Substances Q3A (R2). ICH Harmonised Tripartite Guideline. ICH
Hendrix C, Roets E, Bervoets V, Thomas J, Pijcke M, Busson R, Janssen G, Hoogmartens J (1994) Synthesis of potential impurities of cefalexin and cefradine. Arch Pharm 327:215–219
Olsen BA, Baertschi SW, Riggin RM (1993) Multidimensional evaluation of impurity profiles for generic cefalexin and cefaclor antibiotics. J Chromatogr 648:165–173
Dinner A (1977) Cephalosporin degradations. J Med Chem 20(7):963–965
Tsuji A, Nakashima E, Deguchi Y, Nishide K, Shinizu T, Horiuchi S, Ishikawa K, Yamana T (1981) Degradation Kinetics and mechanism of aminocephalosporins in aqueous solution: cefadroxil. J Pharm Sci 70(10):1120–1128
British Pharmacopeia (BP) (2019) Cefalexin and Cefalexin Cápsules
Bundgaard H (1977) Isolation and characterization of cefalexin degradations products formed in neutral aqueous solution. Arch Pharm Chem Sci Ed 5:149–155
Foog AG, Fayad NM (1979) Differential pulse polarographic determination of Cephalosporins and their degradation products. AnalyticaChimicaActa 108:205–211
United States Pharmacopeia (USP) (2020) Cefalexin and Cefalexin Capsules. USP 43 NF38
European Pharmacopoeia (Ph. Eur.) (2019) 10th edn
Gawande VT, Bothara KG, Marathe AM (2017) Stress studies and identification of degradation products of cefalexin using LC-PDA and LC-MS/MS. Chromatographia 80:1545–1552
Jeswani RM, Sinha PK, Topagi KS, Damle MC (2009) A validated stability HPTLC method for determination of cefalexin in bulk and pharmaceutical formulation. Int J Pharm Tech Res 1:527–536
Reddy MRP, Sreeramulu J, Naidu PY, Reddy AR (2010) Stability indicating fast LC method for the estimation of impurities in cefalexin for oral suspension. Asian J Research Chem 3:1090–1094
Panda SS, Kumar RVVB (2013) Determination of cefalexin monohydrate in pharmaceutical dosage form by stability-indicating RP-UFLC and UV spectroscopy methods. Sci Pharm 81:1029–1041
Rao AL, Prasanthi T, Spandana US (2017) Stability indicating RP-HPLC method development and validation for the analysis of cefalexin and bromhexine in pharmaceutical dosage form. Int J Res AYUSH Pharmac Sci 137–147
ICH Q2 (2005) Validation of analytical procedures. International Conference on Harmonization
ANVISA (2017) Agencia Nacional de Vigilancia Sanitaria, Guide for validation of analytical methods. Resolution of the Collegiate Board of Directors (RDC) n. 166
WHO Expert Committee on Specifications for Pharmaceutical Preparations (October 2005) 40th Report, Technical Report Series 937, Genova 2006, p 12. http://www.who.int/medicines/publications/pharmprep/TRS_937.pdf
Karlesa A, Vera GAD, Dodd MC, Park J, Espino MPB, Lee Y (2014) Ferrate (VI) oxidation of β-lactam antibiotics: reaction kinetics, antibacterial activity changes, and transformation products. Environ Sci Technol 48:10380–10389
Dudkowska J, Franska M, Franski R (2018) Detection of iron complexes with hydrolysis products of cefalexin and cefradine upon high-performance liquid chromatography/electrospray ionization mass spectrometry analysis. Rapid Commun Mass Spectrom 32:576–582
Baertschi SW, Alsante KM, Reed RA (2011) Pharmaceutical stress testing: prediting drug degradation. Informa Healthcare, UK
Acknowledgements
This work was partially financed by CNPq (Project # 310408/2019-9).
Funding
This work was funded by CNPq (Grant no. 310408/2019-9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
von Ahn, A., Dallegrave, A. & dos Santos, J.H.Z. Evaluation of the Cefalexin Drug Degradation Profile in Pharmaceutical Capsule Forms Based on Forced Degradation Studies. Chromatographia 85, 263–279 (2022). https://doi.org/10.1007/s10337-022-04134-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04134-2