Abstract
The influence of cobalt, copper, iron(III), manganese and zinc nitrate salts on phase transitions and thermal stability of ammonium nitrate (AN) has been studied and discussed. Differential thermal analysis/differential scanning calorimetry coupled with thermogravimetry and mass spectrometry were used to evaluate the stability of analyzed systems. Each nitrate salt was appropriately mixed with ammonium nitrate to create samples with AN:salt mass ratios of 4:1, 9:1 and 49:1. It was concluded that the addition of every studied nitrate influenced phase transitions of AN. Most analyzed salts decreased the stability of AN by accelerating its exothermic decomposition process. Iron and cobalt nitrates were defined as the most hazardous additives, resulting in a creation of a highly destabilized mixture. Copper and manganese nitrates were also defined as catalysts of the AN decomposition process, lowering the initial decomposition temperature and increasing the rate of the observed process. Zinc nitrate hexahydrate was the only salt considered to be relatively neutral in such systems, especially in small amounts. The study allowed to define the influence of selected metal nitrate salts on the thermal stability of AN under conditions that are considered as potentially unsafe for such systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ammonium nitrate (AN) is a substance of high importance in the mineral fertilizer industry. Due to its high nitrogen content available to plants in two forms (ammonium—NH4+ and nitrate—NO3−), excellent solubility in water and a simple manufacturing process, AN is commonly used as an inorganic fertilizer [1,2,3]. However, this substance has a tendency to undergo a rapid exothermic decomposition that may result in a detonation when heated or upon impact. Ammonium nitrate has strong oxidizing properties and decomposes almost exclusively into gaseous products, making it a valid and innovative chloride-free propellant and an explosive agent used in mining [4,5,6,7]. Furthermore, its strong hygroscopicity causes excessive caking of fertilizer granules during storage and transportation [8].
AN is used as the main ingredient in CAN, UAN and NPK fertilizers [9]. There is an evident tendency for mineral fertilizer manufacturers to include micronutrient-enriched fertilizers in their product portfolio. Numerous researchers have argued that even small amounts of micronutrients, such as boron, copper, iron, manganese and zinc, have a significant impact on proper and healthy plant growth. They affect crop quality and increase total yield. In addition, an optimal concentration of selected micronutrients can protect the plant from various diseases, such as chlorosis. Even other metals, such as cobalt, chromium or selenium, can be considered beneficial if supplied to plants in an appropriate amount [10,11,12,13].
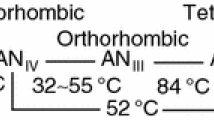
Ammonium nitrate has several known crystal structures that are dependent on temperature, pressure and other various factors, e.g., moisture content. Six crystalline phases have been defined to exist under standard pressure conditions. Table 1 contains known temperature ranges of five ammonium nitrate phases with a defined structure [14,15,16].
The uncontrolled decomposition of ammonium nitrate is considered as an extremely hazardous process. Since the invention of the Haber–Bosch process of ammonia synthesis, there have been reports of numerous disasters resulting from explosions of systems containing ammonium nitrate. Circumstances of these accidents are remembered in the history of chemistry and humanity as particularly tragic events because of the number of victims and value of property damage caused by the self-accelerating explosive decomposition of ammonium nitrate. The most well-known incidents include explosions that occurred on following dates:
-
On September 21, 1921, in Oppau, Germany—a mixture of ammonium nitrate and ammonium sulfate exploded after small charges of dynamite were used to loosen the caked fertilizer mass, resulting in 561 killed and 1952 injured people,
-
On April 16, 1947, in Texas City, USA—a cargo ship, containing ammonium nitrate, caught fire which was tried to be extinguished by filling cargo rooms with large amounts of steam, resulting in explosions of two ships and causing at least 581 fatalities,
-
On December 13, 1994, in Port Neal, USA—a massive explosion occurred at an ammonium nitrate plant due to “a direct result of unsafe operating procedures and conditions”, causing 4 deaths and injuring 18 people,
-
On September 21, 2001, in Toulouse, France—an explosion occurred in a nitrogen fertilizer factory due to a chloride contamination, resulting in 30 killed and 2242 injured people,
-
On April 17, 2013, in West, USA—an ammonium nitrate explosion occurred on site of the fertilizer company, which was caused by a fire of unknown origin, 15 fatalities and over 200 injured were reported,
-
On August 12, 2015, in Tianjin, China—the suspected cause of the fire was the autocatalytic decomposition of nitrocellulose that resulted in the explosion of ammonium nitrate that was stored nearby, killing 173 and injuring almost 800 people,
-
On August 4, 2020, in Beirut, Lebanon—a catastrophic explosion occurred in a warehouse located near the residential district, as a large mass of AN was stored and neglected for years before the incident happened, resulting in 204 fatalities and over 7000 injured people [17,18,19,20,21,22].
Despite numerous studies and expert opinions, accidents of uncontrolled, self-accelerated explosive decomposition of ammonium nitrate have still been observed in recent years, such as disasters that occurred in Tianjin or Beirut. Due to the fact that a universal decomposition inhibitor of AN has not yet been discovered, investigations of the thermal stability of ammonium nitrate systems with various additives are still being considered as necessary [23, 24].
As a result of the potential application of AN in mineral fertilizers, the safety during production, transportation and storage processes is a significant topic. Studies on the thermal stability of ammonium nitrate under various conditions have been conducted by many researchers. Currently, there are several considered decomposition mechanisms described in the literature; however, all of them are hypothetical and exact reaction pathways have yet to be defined. Establishing a consistent description of ammonium nitrate decomposition has been extremely difficult because of many factors that influence the process, such as temperature, pressure, heating rate or the presence of impurities in the system. Presently, it is considered that the first stage of the AN decomposition is an endothermic dissociation to ammonia and nitric acid that occurs above 169 °C (reaction 1).
The exothermic effect of the decomposition of AN is associated with the occurrence of secondary reactions of a parallel and subsequent type caused by products of the endothermic dissociation. Above 290 °C is believed to proceed according to a set of irreversible exothermic reactions that are presented below (reactions 2–6). Depending on the surface-to-volume ratio, the decomposition may be dominated by the endothermic evaporation [8, 25,26,27,28].
The effect of various chemical compounds on the thermal decomposition of AN has been studied and presented in many scientific papers. A group of substances that increase the intensity of the exothermic decomposition includes chlorides, most of inorganic acids and organic compounds, metal-containing compounds and many more. Chlorides have a significant impact on the thermal stability of ammonium nitrate, because even their trace amount can reduce the temperature of its decomposition by up to 70 °C and increase its flammability and explosiveness, intensifying the exothermic effect obtained during decomposition [29,30,31,32,33,34,35]. The previously studied influence of selected metal-containing additives is presented in the discussion part of this study, to allow for an easy comparison between literature and obtained results.
The current knowledge suggests that most carbonates, sulfates, phosphates and potassium salts can increase the thermal stability of AN and partially inhibit the decomposition process [1, 16, 36,37,38,39]. There are many other potential additives with a positive effect on phase transitions that, despite their advantages, cannot be used because they destabilize the product by lowering the start temperature of the decomposition or increasing the susceptibility to transfer the detonation. These are mainly oxides, sulfides or sulfates of aluminum, chromium, copper, iron and nickel [40].
Because ammonium nitrate is also commonly considered as a valid component of studied propellant and energetic systems, numerous studies regarding the enhanced properties of multicomponent systems containing AN have been performed. Co-crystallization technology has been considered promising in recent years, especially since it is possible to include additional catalysts to better optimize desired properties of such systems [4, 7, 41,42,43,44,45].
The aim of this study was to analyze the effect of selected micronutrients (cobalt, copper, iron, manganese and zinc) as nitrate salts on phase transformations and the decomposition process of AN under limited mass transfer conditions that are supposed to simulate hazardous conditions of production and storage of large masses of fertilizer. Until now, only limited, general information regarding the influence of selected metal sources on the thermal stability of AN has been presented by multiple research groups. Majority of performed studies define the inhibiting or catalytic effect of these additives on the endothermic decomposition of ammonium nitrate. Investigations under conditions of limited mass exchange allow to see the practical influence of a given micronutrient on the thermal stability of ammonium nitrate. During its decomposition, the generated gas phase plays a key role in any reactions that occur in studied systems. Using an open system makes it impossible to observe the interaction of the produced gas phase with the micronutrient compound contained in the system. Currently, products containing ammonium nitrate and micronutrient additives are available on the fertilizer market, although studies on thermal stability and process safety are not available in the literature. This work provides a basis for further research on the safety of using AN and its mixtures with other micronutrient sources used in agriculture.
Experimental
Materials
In the study, fertilizer-grade ammonium nitrate was used. AN was obtained directly after the neutralization step and delivered by one of the domestic nitrogen fertilizer producers. Nitrate salts, i.e., cobalt nitrate hexahydrate (CHEMPUR, purity ≥ 99.0 mass%), copper nitrate hemi(pentahydrate) (Honeywell Fluka, purity ≥ 98.0 mass%), iron(III) nitrate nonahydrate (Sigma-Aldrich, purity ≥ 98.0 mass%), manganese nitrate hydrate (Aldrich Chemistry, purity ≥ 98.0 mass%) and zinc nitrate hexahydrate (Sigma-Aldrich, purity ≥ 99.0 mass%) were used without further purification.
An agate mortar and pestle were used for the preparation of each test sample. Adequate amounts of each substance, necessary to acquire a desired mass ratio in a sample (AN:nitrate salt equal to 4:1, 9:1 and 49:1), were ground together until the obtained mixture was considered homogenic. Subsequently, a sample containing approximately 20.0 mg of AN was weighted and placed in a 0.3 cm3 alumina crucible and closed with a pierced lid for DTA-TG measurements. Pure substances were also studied with the use of DSC-TG and masses of approximately 10.0 mg were chosen for the experiment with a 0.085 cm3 alumina crucible without a lid.
Methodology
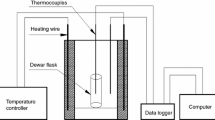
Measurements were performed with the use of differential thermal analysis or differential scanning calorimetry coupled with thermogravimetry and mass spectrometry (DTA/DSC–TG–MS). A thermal analyzer STA 449 F3 with a thermobalance and mass spectrometer QMS 403 C, Netzsch, were used.
The choice of the correct methodology was essential to properly define the influence of selected nitrate salts on the thermal stability of ammonium nitrate. As proven by multiple research groups, the decomposition of AN is strongly influenced by heat and mass transfer conditions, the heating rate, atmosphere of the measurement and mass of the sample [9, 32, 36]. Synthetic air has been chosen as a purge gas because it is the most common atmosphere used in AN storage. Whenever the thermal stability of AN-based systems has to be examined, it is vital to simulate exothermic reactions that occur during the decomposition of AN. This is a very significant detail, as these processes are the main cause of multiple disasters that have occurred over past years. It has been underlined in a previous study [46] that the high value of the surface-to-volume ratio may cause the dissociation process to dominate the decomposition and an endothermic peak to be observed during a thermal analysis measurement. It should always be taken under consideration, unless the precise objective of the performed study is to define the influence of used additives on the dissociation reaction. It was decided that the use of DTA-TG and appropriate AN mass allowed to record the exothermic peak during the ammonium nitrate decomposition process. This may be defined as the most precise simulation of a typical decomposition that causes an explosion of large masses of ammonium nitrate that simultaneously allows to monitor gaseous products of the studied process.
The equipment was calibrated prior to the analysis of studied systems and the procedure was performed according to the producer’s guidelines and consisted of appropriate temperature programs set for indium, tin, bismuth, zinc, aluminum and gold. The heating rate was set to 5 °C min−1 with a 30 cm3 min−1 total flow of the purge gas to simulate conditions of the planned program for ammonium nitrate measurements. Each thermal analysis was evaluated afterward, and correct temperature and sensitivity calibration files were created.
Samples were heated to 450 °C at 5 °C min−1 with a total purge gas flow equal to 30 cm3 min−1. Before each measurement, an empty crucible was heated to 600 °C to remove any remaining possible impurities and a correction to 500 °C was performed to compensate for thermal effects associated with properties of the crucible. Before each measurement, the furnace chamber was evacuated three times and filled with synthetic air. Measurements were performed three times to validate the repeatability of obtained curves. Obtained results were analyzed with the use of professional software supplied by the manufacturer of the measuring equipment. The selected ion monitoring for mass-to-charge ratios (m/z) consisted of following signals: 15 (NH3), 18 (H2O), 30 (NO, NO2), 44 (N2O) and 46 (NO2). DSC-TG and DTA-TG results for pure nitrate compounds were presented as separate figures.
Results and discussion
Ammonium nitrate
Whenever the thermal stability and safety of ammonium nitrate systems are studied with the use of thermal analysis, multiple varying methods are used during the assessment process. General trends tend to focus more on parameters such as the temperature of the start of the decomposition, temperature of the exotherm maximum, temperature range of the decomposition, kinetic parameters of the process and total heat generated during the decomposition [28, 35, 37, 47]. In addition to thermal stability and reactivity, there are also so-called physical parameters that are considered in the safety estimation of production processes and transportation/storage of AN-based fertilizer products. These are the existence or temperature changes of phase transitions from the IV to III and II crystallographic phases, as they result in a significant volume change of the granulated product and lead to an increase in porosity and worsened durability of the fertilizer. All these parameters describing the decomposition process are often the main focus during the research stage as they allow to create a relative guidance on the safe temperature limit that should not be exceeded during the manufacturing process and to evaluate the reaction rate for any safety protocols to be followed in case the temperature is exceeded. In this work, the temperature of the beginning of the decomposition process was defined as the temperature at which signals for produced gaseous products were first recorded and the rate of mass loss achieved the value of 1 mass% of AN contained in the sample per minute.
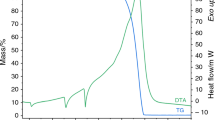
In order to show distinctive differences between the endothermic and exothermic decomposition of AN, both DTA-TG and DSC-TG measurements are shown in Fig. 1a. Since the exothermic decomposition is the main focus of this study, only DTA-TG results were described in detail and included in Table 2 containing all parameters used for the thermal stability assessment of analyzed samples. Even though the exothermic decomposition process of ammonium nitrate has already been defined in previous work [46, 48], authors deemed it necessary to provide an extensive discussion of obtained results to provide readers with all crucial information. As the thermal stability assessment is mainly comparative and the pure AN sample is used as a reference, DTA-TG and MS results obtained during the measurement of the pure AN sample were described below and presented in Fig. 1.
During the heating program, ammonium nitrate underwent a sequence of endothermic phase transformations typical for the compound. Phase transitions IV→III and IV→II were recorded at approximately 52 °C. This atypical crystallographic transformation is often observed when ammonium nitrate is very dry. The II→I crystallographic transformation was observed at around 125 °C and the sample began to melt slightly below 169 °C. The decomposition occurred in the range from 235 to 299 °C, resulting in a complete loss of the sample mass. The exotherm maximum was obtained at almost 285 °C. Because the pierced lid was used, a strong and sharp exothermic signal of the decomposition process was registered on the DTA curve. Mass spectrometry results confirmed the presence of gaseous products (H2O, NH3, NOx and N2O) during the whole process. The comparison with the mostly endothermic decomposition, registered during the DSC-TG measurement of the pure AN sample, showed that the exothermic process began at a higher temperature and was characterized as more rapid than the endothermic dissociation of ammonium nitrate. Obtained results clearly indicate that the DTA-TG method was the better method to use in order to properly assess the thermal stability of AN containing systems in terms of risk and hazard estimation.
Cobalt nitrate hexahydrate systems
DSC-TG and DTA-TG measurements of pure cobalt nitrate hexahydrate are shown in Fig. 2a. It began melting around 47 and 53 °C in the open and semi-closed system respectively, with dehydration happening from the beginning of the analysis up to almost 200 °C for the closed system and 175 °C in the case of an open crucible. The dehydration processes showed visible steps for a sample without a lid that could limit the mass transfer conditions and a single step for a semi-closed system, indicating that a limiting factor was the rate of H2O leaving the crucible. A two-step decomposition began after the dehydration process for the sample undergoing DTA-TG analysis, while a three-step decomposition was visible for the sample in the open crucible. The first step was related to the generation of H2O, NO2 and small amounts of N2O, while the second step, starting at around 235 °C, was characterized by the creation of various nitrogen oxides. Decomposition temperatures of the sample in the open crucible were significantly lower, as the last stage of the process began around 219 °C. Obtained results are similar to or slightly more detailed than findings reported in previous studies of the thermal decomposition of cobalt nitrate, and the remaining black residue could only be defined as Co3O4 [49,50,51].
The influence of cobalt compounds, such as CoZnO and CoFe2O4, was previously studied by other research groups. Both studies defined the influence of the cobalt compound on the thermal decomposition of AN as catalytic; however, the oxide catalyzed the endothermic decomposition process and, in the ferrite study, the obtained exothermic signals were peculiar at best. These studies also used compounds that contained other metals as well, so the individual influence of cobalt could not have been defined. Nonetheless, cobalt compounds used in both studies were the strongest catalysts among other analyzed metal-containing compounds [44, 52]. A similar effect has been reported by Skordilis and Pomonis [53], however, the system containing cobalt was not the least stable. All discussed studies have made cobalt containing compounds to be generally regarded as highly destabilizing and hazardous in mixtures with ammonium nitrate.
Thermal analyses of studied mixtures are presented in Fig. 2b and c. The addition of cobalt salt removed the presence of AN phase transitions in the system, probably due to the creation of double salts or complexes, which seems to be quite typical for systems containing ammonium nitrate with other nitrate compounds. Even endothermic signals for the melting of both nitrates are barely or not visible at all for the mixture containing 20 mass% of the used additive. The exothermic decomposition of the 4:1 sample had a very rapid and sudden progression and the thermal effect could be defined with a sharp exotherm with a maximum at around 285 °C. Gases generated during the observed process consisted of H2O, N2O and NOx. Results obtained during the MS analysis of the 4:1 sample are presented in Fig. 2d. The decomposition process could not be divided into steps, as the steep mass loss on the TG curve occurred in a narrow temperature range of about 48 °C from the beginning to the end of the process, what was the narrowest temperature range of the decomposition process of any AN system studied in this work. Although the initial temperature of the beginning of the decomposition remained relatively typical for AN-based systems, the exothermic process quickly accelerated and could be defined as being more volatile than that for pure ammonium nitrate. Small amounts of cobalt nitrate hexahydrate in mixtures with AN caused the additional endothermic effect to appear at around 117 °C and the decrease in the heat of the melting process. For lower amounts of cobalt nitrate hexahydrate addition to AN (9:1 and 49:1), it was possible to monitor two steps of the decomposition process that are visible on the DTA curve. The second step did not result in the generation of the N2O gas product. The lower the amount of added cobalt nitrate hexahydrate in the heated system, the higher the temperature of the second step of the catalyzed decomposition, and the smaller the effect of the additive on the shape of obtained DTA signal. The most obvious conclusion drawn from studied systems’ decomposition processes is that the addition of cobalt salt to pure AN worsens the thermal stability of the obtained mixture, resulting in systems that may be considered as more dangerous and unstable than typical ammonium nitrate products. Both the high-temperature production process and storage of such products containing cobalt would require additional safety protocols, as the exothermic decomposition of analyzed mixtures is extremely rapid and may result in hazardous explosions that are highly difficult to control.
Copper nitrate hemi(pentahydrate) systems
Pure copper nitrate hemi(pentahydrate) underwent dehydration with simultaneous melting at around 115 °C. For an open system, the decomposition process started at the temperature of the melting, while in a semi-closed system it was defined that above 160 °C the decomposition began. It could be divided into 3 main steps and the final product was CuO. The literature suggests that nitrate and hydroxide double salts can be created during the observed process, as well as copper hydroxide nitrate (Cu2(OH)3(NO3). H2O, N2O and NOx were main gaseous products of the dehydration and consecutive decomposition process. Obtained results are shown in Fig. 3a. Similarly to cobalt nitrate, the thermal decomposition of pure copper nitrate hemi(pentahydrate) occurred in accordance with previously reported studies for pure copper nitrate salts [50, 51, 54, 55].
Likewise previously described cobalt compounds, CuZnO and CuFe2O4 were also analyzed in systems containing ammonium nitrate and defined as strong catalysts of the decomposition process. Among Co, Cu, Mg and Zn ferrites, the copper-containing material was second only to the cobalt compound [42, 43]. Other research groups analyzed the influence of CuO, Cu(NO3)2 and Cu(NO3)2·3Cu(OH)2 on the thermal stability of ammonium nitrate, defining all substances as catalysts of the decomposition process. One of suggested pathways of the catalyzed decomposition includes a formation of diamine copper nitrate complex [53, 56,57,58].
Ammonium nitrate and copper nitrate hemi(pentahydrate) mixtures were analyzed in mass ratios of 4:1, 9:1 and 49:1. Obtained results allowed to observe that the more copper nitrate in the system, the less visible any phase transitions during heating of the sample, including melting of both components. The phenomenon was similar to the one described during the analysis of cobalt mixtures. The possible creation of [Cu(NH3)2](NO3)2 might have been the cause; however, previous studies only confirmed the creation of the complex at elevated temperatures. This might indicate either the presence of a double salt or a lower creation temperature of beforementioned diamine copper nitrate. The mass loss at lower temperatures was caused by the evacuation of water from the system and no visible decomposition of copper nitrate began above 160 °C, which indicated that all the additive had already reacted into a different compound, that was more thermally stable than pure Cu(NO3)2, in all tested samples. Lesser amounts of the additive in the mixture resulted in more visible AN phase transitions, but even with 2 mass% of copper nitrate in the system, the melting started at lower temperature of around 164 °C and had a smaller thermal effect than the melting of the pure AN sample. The main exothermic effect of the decomposition process is strongly shifted toward higher temperatures. The decomposition of studied mixtures could be divided into two separate steps, the first being less intensive, and the second resulting in a sharp exothermic peak. During the less intensive process, the creation of H2O, N2O and NH3 predominated, while the creation of NO2 was the highest during the second, rapid step. The ratio between two exothermic signals depended on the amount of the copper compound in tested systems, as the 49:1 sample was characterized with the main step being the first, less rapid one, while 4:1 and 9:1 samples had a sharp exothermic effect during the second step of the decomposition process with its maximum at around 296 and 300 °C, respectively. Even though the main heat generation was shifted toward higher temperatures in samples containing 20 and 10 mass% of copper nitrate, the decomposition began earlier than in the case of pure ammonium nitrate and studied mixtures were definitely less stable than AN without any additives. Obtained results allowed to determine that copper nitrate hemi(pentahydrate) substantially destabilizes ammonium nitrate and should not be used in production processes that involve elevated temperatures. TG and DTA results are presented in Fig. 3b and c with MS signals for the 4:1 sample shown in Fig. 3d.
Iron nitrate nonahydrate systems
Pure iron nitrate nonahydrate began to melt at around 43 °C in the open system and at 49 °C when the lid was used. During the DSC-TG analysis, it started to dehydrate at the beginning of the measurement until it reached 87 °C. The dehydration process for the semi-closed system was prolonged and lasted until around 127 °C. For both samples, the decomposition process began with the second simultaneous dehydration step, since the MS analysis showed the generation of H2O and NOx. The rapid decrease in mass ended after the system reached approximately 165 °C in both cases. Surprisingly, the decomposition process in open systems seemed to be significantly more complicated than in a semi-closed system, indicating that the possible abundance of air from the purge gas strongly influenced the mechanism of the decomposition process. A slow mass decrease continued to 300 °C. As previous studies have shown, the final residual product of the decomposition was Fe2O3 [50, 59, 60]. DTA-TG and DSC-TG curves for iron nitrate nonahydrate samples are presented in Fig. 4a.
The influence of iron compounds on the thermal stability of ammonium nitrate has been investigated multiple times, as iron is both a micronutrient used in fertilizer production and a very common contaminant. Systems containing AN with iron(III) oxide, rust, pyrite, iron(III) nitrate or iron(III) sulfate were studied by various scientists and all substances were defined as catalysts of the AN thermal decomposition [34, 61,62,63].
DTA-TG and MS results obtained during measurements of systems containing iron are shown in Fig. 4a–c. DTA-TG measurements of ammonium nitrate-iron nitrate nonahydrate systems indicated the creation of double salts or complexes, as typical endothermic phase transitions of AN were significantly less visible compared to pure AN and there was no heat effect connected with the melting of the 4:1 sample. 9:1 and 49:1 samples were characterized with a lesser endothermic effect and a lower temperature of the melting process of around 161 and 168 °C, respectively. The dehydration process consisted of two steps, further proving that iron nitrate nonahydrate cannot be characterized with a single-step dehydration. The decomposition of studied samples was connected with the highest variance of all systems defined in this work and it was difficult to obtain precisely repeatable results, even when multiple analyses of the systems were carried out. It could indicate that such processes consist of multiple competitive reactions that can be strongly influenced by temperature gradients present in used DTA-TG crucibles. The general tendency of observed decomposition processes was that 9:1 samples had the lowest temperature of the end of mass loss, caused by the higher rate of reactions that occurred during the process. The decomposition process was the most complicated of all mixtures of AN with other nitrates. Analysis of TG and DTA curve shapes, together with obtained MS results, indicated that the decomposition process consisted of at least three steps. The first one initiated the decomposition and lasted to approximately 238 °C, with N2O being generated even before the temperature of 180 °C was achieved by the 4:1 sample. Afterward, two simultaneous reaction mechanisms were observed that lasted until the decomposition was complete. The second process did not generate NO2. Observed thermal decompositions of AN-iron nitrate mixtures generated higher amounts of heat and began at temperatures lower than those of their respective analogues described for copper and cobalt systems. The creation of nitrogen oxides was recorded at much lower temperatures (below 180 °C for N2O and below 210 °C for NOx), further indicating the catalytic effect of the added compound. The processes also occurred over a wider range of temperatures. As it stands, the increased variability of the catalyzed decomposition process has to be classified as a highly negative aspect of studied systems, hence the iron nitrate nonahydrate should be defined as a compound that strongly destabilizes AN.
Manganese nitrate hydrate systems
DSC-TG and DTA-TG analyses of pure manganese nitrate hydrate are presented in Fig. 5a. Both samples melted at temperatures lower than the starting temperature of the heating program. The dehydration process lasted until 130 and 150 °C for DSC and DTA measurements, respectively. On the basis of generated TG curves, the studied nitrate salt could probably be defined as a tetrahydrate. After that temperature was reached, the decomposition process began as the simultaneous dehydration continued. Observations of obtained TG curves allowed to conclude that the decomposition process consisted of two stages of the competitive type, as mass transfer conditions in studied systems influenced values of total mass decrease in studied samples and contribution of both steps. After a temperature of around 183 °C for open and 195 °C for semi-closed systems was achieved, all the water was evaporated from the sample and the last stage of the non-accelerated decomposition began and lasted until about 245 °C. MnO2 was the main final product of the decomposition; however, there might have been small amounts of Mn2O3 present in the residue after the DTA-TG measurement, as the final mass loss of the sample was greater than during the DSC-TG analysis. Obtained results were mainly consistent with information given in the literature [50, 51].
The influence of manganese compounds on the thermal stability of AN has, surprisingly, been studied quite scarcely. Skordilis and Pomonis studied the system of manganese nitrate and ammonium nitrate obtained by first dissolving chosen salts in water and then drying the obtained gel for 24 h at 105 °C [53]. It must have been slightly erroneous to assume a prefect ratio in relation to dissolved amounts of salts, as both substances might have decomposed slightly during the drying process. Nonetheless, obtained results indicated that higher amounts of manganese in systems with AN catalyzed the thermal decomposition of the obtained mixture.
It was assumed that double salts or complexes were created after ammonium nitrate and manganese nitrate were mixed, as the disappearance of characteristic phase transitions of AN and the decomposition of manganese nitrate hydrate were observed, as was the case for other previously described systems. DTA-TG curves obtained during measurements of 4:1, 9:1 and 49:1 samples are presented in Fig. 5b and c. The steady mass decrease, lasting up to about 230 °C, was caused by the release of water contained in the manganese salt. Only two endothermic signals recorded for the 4:1 mixture were visible at temperatures corresponding to AN phase transitions, IV and III→II at around 51 °C and the II→I at above 125 °C; however, the second endothermic effect was significantly smaller than for the pure AN. The exothermic decomposition process of the 4:1 sample started at the highest temperature of all systems studied during this research, while the temperature range of the decomposition was narrower than most, second only to cobalt mixtures. The observed decomposition of the studied 4:1 mixture could be separated into two stages, the initial stage with the creation of H2O, N2O and NOx, with N2O being the dominant product, and the second, more rapid and intensive stage that resulted in a sharp exothermic peak on the DTA curve and the creation of large amounts of H2O and NOx, mostly containing NO2 and possibly NO. Lessening the amount of manganese nitrate in the system caused the shift in proportions between two discussed decomposition stages—the initial one became more dominant as the mass ratio of AN to the additive increased. Recorded gaseous products that escaped the furnace during heating of the 4:1 sample are presented as the MS graph in Fig. 5d. It is apparent that the addition of manganese to ammonium nitrate caused the system to become more unstable and hazardous, hence the manganese nitrate hydrate should be considered as a catalyst of the AN decomposition process.
Zinc nitrate hexahydrate systems
The thermal analysis of pure zinc nitrate hexahydrate showed that the salt melted at around 35 °C in the semi-closed system and at 29 °C in the open system. The relatively slow dehydration began immediately after the measurement began, and its dependence on the mass transfer conditions was similar to that of the rest of studied nitrates. The dehydration process lasted until the system reached a temperature of 260 °C and the simultaneous exothermic decomposition began, occurring until a temperature of around 340 °C was achieved. Obtained results are presented in Fig. 6a. The final product of the decomposition process was defined as ZnO by other research groups in their studies [50, 51, 64].
Zinc compounds in systems with AN have been studied to a limited extent in the form of ZnFe2O4, Zn(NO3)2, ZnSO4 and ZnO, but only limited data are currently available and no extensive description was given in any previously written manuscript [52, 61].
Results of DTA-TG-MS measurements of systems containing AN and the zinc compound are shown in Fig. 6b–d. As observed for samples containing 20 mass% of zinc nitrate hexahydrate, the formation of double salts or complexes was apparent from results obtained on the DTA curve. Only the first phase transition, which occurs slightly above 51 °C, was typical for pure AN and the endothermic effect of melting was not visible at all. That might have indicated that the mixture becomes reactive at temperatures above the temperature of the first phase transition. Since the decomposition process of zinc nitrate begins above 260 °C, it is impossible to define whether the salt decomposes on its own or together with AN. The beginning of the exothermic decomposition process of the 4:1 sample was observed above 230 °C, what is a lower value than the one characteristic for pure AN, but the process itself was significantly less dynamic than the decomposition process of ammonium nitrate or its mixtures with copper, cobalt, iron or manganese nitrate in the same mass ratios. However, in the case of the zinc nitrate additive, N2O was generated at lower temperatures (from approximately 190 °C) than during a typical ammonium nitrate decomposition, indicating that the slow decrease in sample mass mainly resulted from the generation of this product. NOx were also produced above 200 °C at a slow rate. The observed decomposition was also composed of two separate steps, with the second one beginning at around 305 °C and lasting up to the temperature of 318 °C. The second step resulted in the creation of higher amounts of NOx than in the first step, even though only 7 mass% of total mass decreased during this step. When the amount of zinc nitrate hexahydrate in the system is lower, the second step of the decomposition process is more visibly separated from the first one. Even though the heat evolved during the decomposition has been higher for 4:1 and 9:1 samples than in the case of pure AN and the beginning of the decomposition is noticeable at lower temperatures, the influence of zinc nitrate on the thermal stability of ammonium nitrate should not be defined as a negative one. Zinc nitrate hexahydrate creates the least hazardous mixtures with ammonium nitrate in comparison with other studied nitrate salts, by a large margin, in the case of linear heating conditions. Additional isothermal analyzes could be suggested to further assess the possible instability of the system at constant elevated temperatures of 180–200 °C.
Conclusions
The performed research has shown that most of studied d-metal nitrate salts negatively influence the thermal stability of ammonium nitrate. Every additive, when mixed with AN in a significant enough amount, visibly affected the presence of all phase transitions that would be expected in samples containing ammonium nitrate. Substances that decomposed at lower temperatures also did not undergo standard decompositions in systems with AN, indicating the presence of more stable double salts or complexes.
Cobalt nitrate hexahydrate has been proven to be the relatively strongest catalyst of the AN exothermic decomposition among all tested substances. It visibly increased the rate of the observed process and sharpened the shape of the generated DTA curve during the decomposition. Although the amount of generated heat was much lower than in the case of pure AN, the substance could not be considered safe for use in any high-temperature fertilizer production process. Manganese and copper compounds had an effect on AN similar to that of the studied cobalt nitrate and were identified as potent catalysts of the thermal decomposition process. Even small amounts of both additives visibly destabilized analyzed systems.
Iron nitrate nonahydrate had different destabilizing effects on ammonium nitrate. Higher amounts of the additive significantly lowered the temperature of the beginning of the decomposition process and increased the amount of generated heat. Observed decomposition processes of studied systems were more complex and consisted of multiple visible steps. Obtained mixtures also behaved less repeatably, indicating that such systems were more sensitive to the temperature gradient during measurements. Even though observed decomposition processes were not the most rapid of all studied systems, the analyzed iron compound should be considered to be the most hazardous and destabilizing in mixtures with AN among all researched nitrate salts.
Zinc nitrate hexahydrate mixed with ammonium nitrate was the most stable of all studied systems. It should not be considered as an inhibitor of the thermal decomposition of ammonium nitrate, though its influence in higher amounts could be defined as borderline destabilizing, while small amounts might be considered neutral in mixtures with AN.
Presented results only define the thermal stability of samples tested under described conditions and additional studies should be performed if the influence of studied substances is of interest in practical cases not focused on a fertilizer production. Additional measures might have to be taken if any multicomponent fertilizers containing ammonium nitrate and studied metal ions are to be produced. Research on the thermal stability of ammonium nitrate mixtures and risks related to their use and production should be broadly studied and an increase in popularity of this topic is necessary for the safety of chemical engineers and people living near chemical plants and warehouses used for storage of such products.
References
Klimova I, Kaljuvee T, Turn L, Bender V, Trikkel A, Kuusik R. Interactions of ammonium nitrate with different additives. J Therm Anal Calorim. 2011;105:13–26. https://doi.org/10.1007/s10973-011-1514-9.
Aleinov DP. Nitric acid, nitrates, and ammonium salts. In: United Nations Industrial Development Organization, International Fertilizer Development Center (Eds.), Fertilizer manual, 3rd ed. Dordrecht: Kluwer Academic Publishers; 1998, p. 207–253.
Recous S, Machet JM, Mary B. The partitioning of fertilizer-N between soil and crop: comparison of ammonium and nitrate applications. Plant Soil. 1992;144:101–11. https://doi.org/10.1007/BF00018850.
Oommen C, Jain SR. Ammonium nitrate: a promising rocket propellant oxidizer. J Hazard Mater. 1999;A67:253–81. https://doi.org/10.1016/S0304-3894(99)00039-4.
Hadzik J, Koślik P, Wilk Z, Frodyma A, Habera Ł. Experimental study on ammonium nitrate(V)-based solid propellants for fracturing wells. Cent Eur J Energ Mater. 2017;14(3):660–74. https://doi.org/10.22211/cejem/76721.
Asgari A, Ghani K, Keshavarz MH, Mousaviazar A, Khajavian R. Ammonium nitrate-MOF-199: a new approach for phase stabilization of ammonium nitrate. Thermochim Acta. 2018;667:148–52. https://doi.org/10.1016/j.tca.2018.07.018.
Hanafi S, Trache D, Mezroua A, Boukeciat H, Meziani R, Tarchoun AF, Abdelaziz A. Optimized energetic HNTO/AN co-crystal and its thermal decomposition kinetics in the presence of energetic coordination nanomaterials based on functionalized graphene oxide and cobalt. RSC Adv. 2021;11:35287–99. https://doi.org/10.1039/D1RA06367G.
Chaturvedi S, Dave PN. Review on thermal decomposition of ammonium nitrate. J Energ Mater. 2013;31:1–26. https://doi.org/10.1080/07370652.2011.573523.
Popławski D, Hoffmann J, Hoffmann K. Effect of carbonate minerals on the thermal stability of fertilisers containing ammonium nitrate. J Therm Anal Calorim. 2016;124:1561–74. https://doi.org/10.1007/s10973-015-5229-1.
Hu X, Wei X, Ling J, Chen J. Cobalt: An essential micronutrient for plant growth? Front Plant Sci. 2021;12:A768523. https://doi.org/10.3389/fpls.2021.768523.
Lanza M, Reis ARD. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol Biochem. 2021;164:27–43. https://doi.org/10.1016/j.plaphy.2021.04.026.
López-Rayo S, Lucena JJ, Laghi L, Cremonini MA. Demetalation of Fe, Mn, and Cu chelates and complexes: application to the NMR analysis of micronutrient fertilizers. J Agric Food Chem. 2011;59(24):13110–6. https://doi.org/10.1021/jf203602a.
Jie M, Raza W, Xu YC, Shen QR. Preparation and optimization of amino acid chelated micronutrient fertilizer by hydrolyzation of chicken waste feathers and the effects on growth of rice. J Plant Nutr. 2008;31:571–82. https://doi.org/10.1080/01904160801895092.
Dunuwille M, Yoo CS. Phase diagram of ammonium nitrate. J Chem Phys. 2013;139:A214503. https://doi.org/10.1063/1.4837715.
Herrmann MJ, Engel W. Phase transitions and lattice dynamics of ammonium nitrate. Propell Explos Pyrot. 1997;22:143–7. https://doi.org/10.1002/prep.19970220308.
Kiiski H. Properties of ammonium nitrate based fertilisers. Doctoral dissertation, University of Helsinki, Finland, 2009.
Pittman W, Han Z, Harding B, Rosas C, Jiang J, Pineda A, Mannan MS. Lessons to be learned from an analysis of ammonium nitrate disasters in the last 100 years. J Hazard Mater. 2014;280:472–7. https://doi.org/10.1016/j.jhazmat.2014.08.037.
Dechy N, Bourdeaux T, Ayrault N, Kordek MA, Le Coze JC. First lessons of the Toulouse ammonium nitrate disaster, 21st September 2001, AZF plant. France J Hazard Mater. 2004;111:131–8. https://doi.org/10.1016/j.jhazmat.2004.02.039.
Laboureur D, Han Z, Harding B, Pineda A, Pittmann W, Rosas C, Jiang J, Mannan M. Case study and lessons learned from the ammonium nitrate explosion at the west fertilizer facility. J Hazard Mater. 2016;308:164–72. https://doi.org/10.1016/j.jhazmat.2016.01.039.
Yu G, Duh YS, Yang X, Li Y, Chen Y, Li Y, Li J, Chen R, Gong L, Yang B, Huang J. Holistic case study on the explosion of ammonium nitrate in Tianjin Port. Sustainability. 2022;14(6):A3429. https://doi.org/10.3390/su14063429.
Yu G, Wang Y, Zheng L, Huang J, Li J, Gong L, Chen R, Li W, Huang J, Duh YS. Comprehensive study on the catastrophic explosion of ammonium nitrate stored in the warehouse of Beirut port. Process Saf Environ Protect. 2021;152:201–19. https://doi.org/10.1016/j.psep.2021.05.030.
Sivaraman S, Varadharajan S. Investigative consequence analysis: a case study research of beirut explosion accident. J Loss Prevent Proc. 2021;69:104387. https://doi.org/10.1016/j.jlp.2020.104387.
Babrauskas V. Explosions of ammonium nitrate fertilizer in storage or transportation are preventable accidents. J Hazard Mater. 2016;304:134–49. https://doi.org/10.1016/j.jhazmat.2015.10.040.
Marlair G, Kordek MA. Safety and security issues relating to low capacity storage of AN-based fertilizers. J Hazard Mater. 2005;A123:13–28. https://doi.org/10.1016/j.jhazmat.2005.03.028.
Cao H, Jiang L, Duan Q, Zhang D, Chen H, Sun J. An experimental and theoretical study of optimized selection and model reconstruction for ammonium nitrate pyrolysis. J Hazard Mater. 2019;364:539–47. https://doi.org/10.1016/j.jhazmat.2018.10.048.
Skarlis SA, Nicolle A, Berthout D, Dujardin C, Granger P. Combined experimental and kinetic modeling approaches of ammonium nitrate thermal decomposition. Thermochim Acta. 2014;584:58–66. https://doi.org/10.1016/j.tca.2014.04.004.
Vyazovkin S, Clawson JS, Wight CA. Thermal dissociation kinetics of solid and liquid ammonium nitrate. Chem Mater. 2001;13:960–6. https://doi.org/10.1021/cm000708c.
Yang M, Chen X, Wang Y, Yuan B, Niu Y, Zhang Y, Liao R, Zhang Z. Comparative evaluation of thermal decomposition behavior and thermal stability of powdered ammonium nitrate under different atmosphere conditions. J Hazard Mater. 2017;337:10–9. https://doi.org/10.1016/j.jhazmat.2017.04.063.
Rubtsov YI, Kazakov AI, Lempert DB, Manelis GB. Kinetic regularities of the heat release for the interaction of some organic compounds with ammonium nitrate. Propell Explos Pyrot. 2006;31(6):421–34. https://doi.org/10.1002/prep.200600057.
Han Z, Sachdeva S, Papadaki MI, Mannan MS. Effects of inhibitor and promoter mixtures on ammonium nitrate fertilizer explosion hazards. Thermochim Acta. 2016;624:69–75. https://doi.org/10.1016/j.tca.2015.12.005.
Sinditskii VP, Egorshev VY, Levshenkov AI, Serushkin VV. Ammonium nitrate: combustion mechanism and the role of additives. Propell Explos Pyrot. 2005;39(4):269–80. https://doi.org/10.1002/prep.200500017.
Izato Y, Miyake A. Thermal decomposition mechanism of ammonium nitrate and potassium chloride mixtures. J Therm Anal Calorim. 2015;121:287–94. https://doi.org/10.1007/s10973-015-4739-1.
Sun J, Sun Z, Wang Q, Ding H, Wang T, Jiang C. Catalytic effects of inorganic acids on the decomposition of ammonium nitrate. J Hazard Mater. 2005;B127:204–10. https://doi.org/10.1016/j.jhazmat.2005.07.028.
Gunawan R, Zhang D. Thermal stability and kinetics of decomposition of ammonium nitrate in the presence of pyrite. J Hazard Mater. 2009;165:751–8. https://doi.org/10.1016/j.jhazmat.2008.10.054.
Oxley JC, Smith JL, Rogers E, Yu M. Ammonium nitrate: thermal stability and explosivity modifiers. Thermochim Acta. 2002;384:23–45. https://doi.org/10.1016/S0040-6031(01)00775-4.
Yang M, Chen X, Yuan B, Wang Y, Rangwala AS, Cao H, Niu Y, Zhang Y, Fan A, Yin S. Inhibition effect of ammonium dihydrogen phosphate on the thermal decomposition characteristics and thermal sensitivity of ammonium nitrate. J Anal Appl Pyrol. 2018;134:195–201. https://doi.org/10.1016/j.jaap.2018.06.008.
Han Z, Sachdeva S, Papadaki MI, Sam MM. Ammonium nitrate thermal decomposition with additives. J Loss Prevent Proc. 2015;35:307–15. https://doi.org/10.1016/j.jlp.2014.10.011.
Tan L, Xia L, Wu Q, Xu S, Liu D. Effect of urea on detonation characteristics and thermal stability of ammonium nitrate. J Loss Prevent Proc. 2015;38:169–75. https://doi.org/10.1016/j.jlp.2015.09.012.
Kaljuvee T, Edro E, Kuusik R. Influence of lime-containing additives on the thermal behaviour of ammonium nitrate. J Therm Anal Calorim. 2008;92:215–21. https://doi.org/10.1007/s10973-007-8769-1.
Vyazenova IA, Levchenko IV, Taranushich VA, Chernyshev VA. Thermal decomposition of ammonium nitrate with three-component additives. Russ J Appl Chem. 2015;88:574–8. https://doi.org/10.1134/S1070427215040035.
Pandey M, Jha S, Kumar R, Mishra S, Jha R. The pressure effect study on the burning rate of ammonium nitrate-HTPB-based propellant with the influence catalysts. J Therm Anal Calorim. 2012;107:135–40. https://doi.org/10.1007/s10973-011-1718-z.
Kohga M, Okamoto K. Thermal decomposition behaviors and burning characteristics of ammonium nitrate/polytetrahydrofuran/glycerin composite propellant. Combust Flame. 2011;158:573–82. https://doi.org/10.1016/j.combustflame.2010.10.009.
Jisna J, Suresh M. Ammonium nitrate as an eco-friendly oxidizer for composite solid propellants: promises and challenges. Crit Rev Solid State Mater Sci. 2017;42:470–98. https://doi.org/10.1080/10408436.2016.1244642.
Vara JA, Dave PN. Metal oxide nanoparticles as catalyst for thermal behavior of AN based composite solid propellant. Chem Phys Lett. 2019;730:600–7. https://doi.org/10.1016/j.cplett.2019.06.048.
Hanafi S, Trache D, Meziani R, Boukeciat H, Abdelaziz A, Tarchoun AF, Abdelaziz A. Catalytic reactivity of graphene oxide stabilized Fe complexe of triaminoguanidine on thermolysis of HNTO/AN co-crystal. Thermochim Acta. 2022;717:179324–32. https://doi.org/10.1016/j.tca.2022.179324.
Kaniewski M, Hoffmann K, Hoffmann J. Influence of selected potassium salts on thermal stability of ammonium nitrate. Thermochim Acta. 2019;678:A178313. https://doi.org/10.1016/j.tca.2019.178313.
Cao H, Jiang L, Duan Q, Chai H, Li X, Sun J. Experimental study of the effect of typical halides on pyrolysis of ammonium nitrate using model reconstruction. J Hazard Mater. 2020;384:A121297. https://doi.org/10.1016/j.jhazmat.2019.121297.
Hoffmann J, Kaniewski M, Nieweś D, Hoffmann K. Selected magnesium compounds as possible inhibitors of ammonium nitrate decomposition. Pol J Chem Technol. 2020;22(2):1–8. https://doi.org/10.2478/pjct-2020-0011.
Brockner W, Ehrhardt C, Gjikaj M. Thermal decomposition of nickel nitrate hexahydrate, Ni(NO3)2·6H2O, in comparison to Co(NO3)2·6H2O and Ca(NO3)2·4H2O. Thermochim Acta. 2007;456:64–8. https://doi.org/10.1016/j.tca.2007.01.031.
Cseri T, Békássy S, Kenessey G, Liptay G, Figueras F. Characterization of metal nitrates and clay supported metal nitrates by thermal analysis. Thermochim Acta. 1996;288:137–54. https://doi.org/10.1016/S0040-6031(96)03037-7.
Małecka B, Łącz A, Drożdż E, Małecki A. Thermal decomposition of d-metal nitrates supported on alumina. J Therm Anal Calorim. 2015;119:1053–61. https://doi.org/10.1007/s10973-014-4262-9.
Cabrera AF, Rodríguez Torres CE, Juncal LC, Meyer M, Stewart SJ. Effect of nanostructured ferrites MFe2O4 (M=Cu Co, Mg, Zn) on the thermal decomposition of ammonium nitrate. App Energy Combust Sci. 2021;6:A100026. https://doi.org/10.1016/j.jaecs.2021.100026.
Skordilis CS, Pomonis PJ. The influence of Mn, Co and Cu cations on the thermal decomposition of NH4NO3 in pure form and supported on alumina. Thermochim Acta. 1993;216:137–46. https://doi.org/10.1016/0040-6031(93)80387-P.
L’vov BV, Novichikhin AV. Mechanism of thermal decomposition of hydrated copper nitrate in vacuo. Spectrochim Acta B At Spectrosc. 1995;50:1459–68. https://doi.org/10.1016/0584-8547(95)01402-0.
Mansour SAA. Thermoanalytical investigation of the decomposition course of copper oxysalts. II. Copper (II) nitrate trihydrate. J Therm Anal Calorim. 1995;45:1381–92. https://doi.org/10.1007/BF02547432.
Shiota K, Matsunaga H, Miyake A. Thermal analysis of ammonium nitrate and basic copper(II) nitrate mixtures. J Therm Anal Calorim. 2015;121:281–6. https://doi.org/10.1007/s10973-015-4536-x.
Izato Y, Kajiyama K, Miyake A. Thermal decomposition mechanism of ammonium nitrate and copper(II) oxide mixtures. Sci Technol Energetic Mater. 2014;75(5):128–33.
Vargeese AA, Muralidharan K. Kinetics and mechanism of hydrothermally prepared copper oxide nanorod catalyzed decomposition of ammonium nitrate. Appl Catal A Gen. 2012;447–448:171–7. https://doi.org/10.1016/j.apcata.2012.09.027.
Melnikov P, Nascimento VA, Arkhangelsky IV, Zanoni Consolo LZ, de Oliveira LCS. Thermal decomposition mechanism of iron(III) nitrate and characterization of intermediate products by the technique of computerized modeling. J Therm Anal Calorim. 2014;115:145–51. https://doi.org/10.1007/s10973-013-3339-1.
Wieczorek-Ciurowa K, Kozak AJ. The thermal decomposition of Fe(NO3)3·9H2O. J Therm Anal Calorim. 1999;58:647–51. https://doi.org/10.1023/A:1010112814013.
Oxley JC, Kaushik SM, Gilson NS. Thermal stability and compatibility of ammonium nitrate explosives on a small and large scale. Thermochim Acta. 1992;212:77–85. https://doi.org/10.1016/0040-6031(92)80222-I.
Xu Z, Liu D, Hu Y, Ye Z, Wei Y. Influence of iron ion on thermal behavior of ammonium nitrate and emulsion explosives. Cent Eur J Energ Mater. 2010;7(1):77–93.
Oluwoye I, Mosallanejad S, Soubans G, Altarawneh M, Gore J, Dlugogorski BZ. Thermal decomposition of ammonium nitrate on rust surface: risk of low-temperature fire. Fire Saf J. 2021;120:A103063. https://doi.org/10.1016/j.firesaf.2020.103063.
Maneva M, Petrov N. On the thermal decomposition of Zn(NO3)2·6H2O and its deuterated analogue. J Therm Anal Calorim. 1989;35:2297–303. https://doi.org/10.1007/BF01911893.
Funding
This research was funded by the Ministry of Science and Higher Education of Poland within a frame of science subsidy for 2021 which was realized in the Department of Engineering and Technology of Chemical Processes, Wroclaw University of Science and Technology (No. 8201003902-K24W03D05).
Author information
Authors and Affiliations
Contributions
MK: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing—original draft, Writing—Review & Editing, Visualization, Supervision, Project administration; MB: Validation, Formal analysis, Investigation, Data curation, Writing—original draft, Visualization, Writing—Review & Editing; JH: Resources, Supervision, Project administration; Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest was declared during the submission of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaniewski, M., Biegun, M. & Hoffmann, J. Thermal stability of ammonium nitrate systems containing d-metal nitrate salts under limited mass transfer conditions. J Therm Anal Calorim 148, 5309–5323 (2023). https://doi.org/10.1007/s10973-023-12137-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12137-w