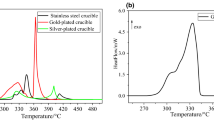
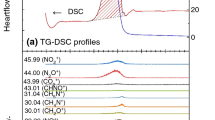
Abstract
The thermal decomposition of ammonium nitrate (AN) and potassium chloride (KCl) mixtures was investigated. The thermal properties were studied using differential scanning calorimetry (DSC), and the evolved gas was analyzed using thermogravimetry with mass spectrometry and pressurized DSC coupled with mass spectrometry (TG–DTA–MS and PDSC–MS). DSC measurements of AN/KCl mixtures in sealed and sealed-separated sample pans showed a sharp exothermic decomposition and lower onset temperature than pure AN, whereas AN/KCl in an open pan exhibited an endothermic reaction. In the sealed-separated pan, a separator with a pinhole divided the KCl and AN physically and the evolved gas could interact with both AN and KCl. TG–DTA–MS results revealed that HCl gas was evolved from AN/KCl, which indicated that the reaction of KCl with HNO3 dissociated from AN formed HCl, and subsequent destabilization of AN. However, the TG–DTA–MS results did not indicate the violent exothermic reaction due to using an open pan and ordinary pressure conditions. PDSC–MS was used to observe two exothermic reactions of AN/KCl and analyze the evolved gases from the reactions. A violent first exothermic reaction was accompanied by a large amount of N2 and N2O gases without H2O, and a second exothermic reaction accompanied by H2O, N2O, and other gases occurred subsequently. The reactions are \( {\text{HCl }} + {\text{HNO}}_{ 3} \to {\text{NO}}_{ 2} {\text{Cl }} + {\text{H}}_{ 2} {\text{O }} \to {\text{Cl }} + {\text{NO}}_{ 2} + {\text{H}}_{ 2} {\text{O}} \), which have a lower energy barrier by 103 kJ mol−1 than the energy barrier that is needed for HNO3 homolysis cleavage, which is triggered by pure AN decomposition, \( {\text{HNO}}_{ 3} \to {\text{OH}} + {\text{NO}}_{ 2} \). We therefore concluded that AN reacts with KCl to produce Cl radicals via HCl and NO2Cl, and the Cl radical triggers a radical chain reaction of AN.







Similar content being viewed by others
References
Oommen C, Jain SR. Ammonium nitrate: a promising rocket propellant oxidizer. J Hazard Mater. 1999;67:253–81.
Oxley JC, Smith JL, Wang W. Compatibility of ammonium nitrate with monomolecular explosives. Part II Nitroarenes. J Phys Chem. 1994;98:3901–7.
Marlair G, Kordek M. Safety and security issues relating to low capacity storage of AN-based fertilizers. J Hazard Mater. 2005;A123:13–28.
Chemical Safety Board. Preliminary Findings of the U.S. Chemical Safety Board from its Investigation of the West Fertilizer Explosion and Fire. 2014. http://www.csb.gov/assets/1/19/West_Preliminary_Fin dings.pdf. Accessed 11 March 2014.
Dechy N, Bourdeaux T, Ayrault A, Kordek M, Coze JL. First lessons of the Toulouse ammonium nitrate disaster, 21st September 2001, AZF plant. France. J Hazard Mater. 2004;111:131–8.
Brower KR, Oxley JC, Tewari MP. Evidence for hemolytic decomposition of ammonium nitrate at high temperature. J Phys Chem. 1989;93:4029–33.
Keenan AG, Notz K, Franco NB. Synergistic catalysis of ammonium nitrate decomposition. J Am Chem Soc. 1969;91:3168–71.
Macneil JH, Zhang H, Berseth P, Trogler WC. Catalytic decomposition of ammonium nitrate in superheated aqueous solutions. J Am Chem Soc. 1997;119:9738–44.
Li XR, Koseki H. Study on the contamination of chlorides in ammonium nitrate. Process Saf Environ Prot. 2005;83(B1):31–7.
Sun J, Sun Z, Wang Q, Ding H, Wang T, Jiang C. Catalytic effects of inorganic acids on the decomposition of ammonium nitrate. J Hazard Mater. 2005;B127:204–10.
Kajiyama K, Izato Y, Miyake A. Thermal characteristics of ammonium nitrate, carbon, and copper (II) oxide mixtures. J Therm Anal Calorim. 2013;113:1475–80.
Oxley JC, Smith JL, Naik S, Moran J. Decompositions of urea and guanidine nitrate. J Energ Mater. 2009;27:17–39.
Izato Y, Date S, Miyake A. Combustion characteristics of ammonium nitrate and carbon mixtures based on a thermal decomposition mechanism. Propellants Explos Pyrotech. 2013;38:129–35.
Sinditskii VP, Egorshev VY, Levshenkov AI, Serushkin VV. Ammonium nitrate: combustion mechanism and the role of additives. Propellants Explos Pyrotech. 2005;30:269–80.
Koroban VA. Thermal decomposition features of ammonium nitrate and its boron mixture under high pressures. Propellants Explos Pyrotech. 1994;19:307–10.
Patil DG, Jain SR, Brill TB. Thermal decomposition of energetic materials 56. On the fast thermolysis mechanism of ammonium nitrate and its mixtures with magnesium and carbon. Propellants Explos Pyrotech. 1992;17:99–105.
Miyake A, Izato Y. Thermal decomposition behaviors of ammonium nitrate and carbon mixtures. Int J Energ Mater Chem Propuls. 2010;9:467–75.
Mirvakili A, Samimi F, Jahanmiri A. Simultaneous ammonium nitrate decomposition and NOX emission reduction in a novel configuration of membrane reactor: a simulation study. J Ind Eng Chem. 2014;20:2452–62.
Skarlis SA, Nicolle A, Berthout D, Dujardin C, Granger P. Combined experimental and kinetic modeling approachies of ammonium nitrate thermal decomposition. Thermochim Acta. 2014;584:58–66.
Sinditskii VP, Egorshev VY, Serushkin VV, Filatov SA. Combustion of energetic materials controlled by condensed-phase reactions. Combust Explos Shock Waves. 2012;48:81–99.
Cagnina S, Rotureau P, Fayet G, Adamo C. The ammonium nitrate and its mechanism of decomposition in the gas phase: a theoretical study and a DFT benchmark. Phys Chem Chem Phys. 2013;15:10849–59.
Izato Y, Miyake A, Echigoya H. Influence of the physical properties of carbon on the thermal decomposition behavior of ammonium nitrate and carbon mixtures. Sci Technol Energ Mater. 2009;70:101–4.
Wada Y, Hori K, Arai M. Combustion mechanism of mixtures of guanidine nitrate, ammonium nitrate, and basic copper nitrate. Sci Technol Energ Mater. 2010;71:83–7.
Hasue K, Miura R, Yoshitake K. Equation for burning rate as a function of pressure and temperature for potassium nitrate-phase stabilized ammonium nitrate/1H-tetrazole mixture. Sci Technol Energ Mater. 2013;74:113–7.
Fujisato K, Habu H, Miyake A, Hori K. Thermal decomposition of ammonium nitrate modeling of thermal dissociation in thermal analysis. Sci Technol Energ Mater. 2014;75:28–36.
Ikeda K, Shiraishi Y, Date S. Burning characteristics of some azodicarbonamide/ammonium nitrate/additive mixtures. Sci Tech Energ Mater. 2014;75:59–63.
Ikeda K, Doi A, Date S. Burning characteristics of some azodicarbonamide/ammonium nitrate/additive mixtures (II). Sci Technol Energ Mater. 2014;75:83–5.
Izato Y, Kajiyama K, Miyake A. Thermal decomposition mechanism of ammonium nitrate and copper (II) oxide mixtures. Sci Technol Energ Mater. 2014;75:128–33.
Fenter FF, Caloz F, Rossi MJ. Kinetics of nitric acid uptake by salt. J Phys Chem. 1994;98:9801–10.
NIST chemistry web book. http://webbook.nist.gov/chemistry/. Accessed 3 Sep 2014.
Friedel RA, Shultz JL, Sharkey AG. Mass spectrum of nitric acid. Anal Chem. 1959;31:1128.
Wagman DD, Evans WH, Parker VB, Schumm RH, Halow I, Bailey SM, Churney KL, Nuttall RL. The NBS tables of chemical thermodynamic properties. J Phys Chem Ref Data. 1982;11:67–72.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Izato, Yi., Miyake, A. Thermal decomposition mechanism of ammonium nitrate and potassium chloride mixtures. J Therm Anal Calorim 121, 287–294 (2015). https://doi.org/10.1007/s10973-015-4739-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4739-1