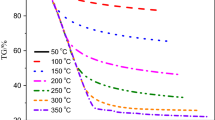
Abstract
Residual oils, high viscosity and large sulfur content petroleum products from the refining process of crude oil, are receiving increasing interest in pre-combustion carbon capture applications. Gasification is a promising technology to convert such complicated hydrocarbons into syngas. Pyrolysis and combustion are very important stages in the gasification process, and therefore a better understanding of these processes leads to higher efficiency and better development of such applications. In this work, pyrolysis and combustion of heavy fuel oil (HFO) and vacuum residual oil (VRO) were studied in a thermogravimetric analyzer (TGA). The HFO studied in this work is a blend of VRO and diesel, which provides insight into the performance of residual oils/diesel blends. The TGA experiments were conducted using nitrogen and mixtures of oxygen and nitrogen for pyrolysis and combustion studies, respectively, at different heating rates (5–20 °C min−1). The oxygen concentration was varied from 0 to 71.4%vol. to replicate oxygen concentration in applications ranging from pyrolysis (0% O2) to combustion (21% O2) and gasification (high O2%). The TGA experiments covered a temperature range from ambient to 1200 °C. The results show that pyrolysis is slightly slower than combustion at low temperatures for both oils. However, pyrolysis is significantly faster at high temperatures. The combustion of both oils resulted in minimal residue, while the residue remaining in the pyrolysis is 10–19%. The TGA was coupled with Fourier transform infrared spectroscopy (FTIR) to monitor the evolved volatiles from the pyrolysis and combustion processes. The results show more aromatics evolved from VRO than HFO. Apparent kinetic parameters were calculated using three model-free methods and a model-based method (Coats and Redfern).




















Similar content being viewed by others
References
Rogelj J, Den Elzen M, Höhne N, Fransen T, Fekete H, Winkler H, et al. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature. 2016;534(7609):631–9. https://doi.org/10.1038/nature18307.
Bos K, Gupta J. Climate change: the risks of stranded fossil fuel assets and resources to the developing world. Third World Q. 2018;39(3):436–53. https://doi.org/10.1080/01436597.2017.1387477.
IEA. Renewable energy market update. Paris IEA 2020.
Osman AI, Hefny M, Abdel Maksoud M, Elgarahy AM, Rooney DW. Recent advances in carbon capture storage and utilisation technologies: a review. Environ Chem Lett. 2021;19(2):797–849. https://doi.org/10.1007/s10311-020-01133-3.
Furimsky E. Gasification in petroleum refinery of 21st century. Oil Gas Sci Technol. 1999;54(5):597–618. https://doi.org/10.2516/ogst:1999051.
Guida P, Jameel AGA, Saxena S, Roberts WL. Fundamental aspects and applications of ultrasonically induced cavitation in heavy fuel oil with a focus on deasphalting, emulsions, and oxidative desulfurization. In: Dalai AK, Dadyburjor DB, Zheng Y, Duan A, Roberts WL, Nanda S, editors. Catalytic and noncatalytic upgrading of oils. ACS Publications; 2021. p. 233–93.
Saadatkhah N, Carillo Garcia A, Ackermann S, Leclerc P, Latifi M, Samih S, et al. Experimental methods in chemical engineering: thermogravimetric analysis—TGA. Can J Chem Eng. 2020;98(1):34–43. https://doi.org/10.1002/cjce.23673.
Wang S, Lu G. Thermogravimetric analysis of carbon deposition over Ni/γ-Al2O3 catalysts in carbon dioxide reforming of methane. Energy Fuels. 1998;12(6):1235–40. https://doi.org/10.1021/ef980064j.
Jameel AGA, Han Y, Brignoli O, Telalović S, Elbaz AM, Im HG, et al. Heavy fuel oil pyrolysis and combustion: kinetics and evolved gases investigated by TGA-FTIR. J Anal Appl Pyrolysis. 2017;127:183–95. https://doi.org/10.1016/j.jaap.2017.08.008.
Ghetti P. A rapid heating TGA method for evaluating the carbon residue of fuel oil. Fuel. 1994;73(12):1918–21. https://doi.org/10.1016/0016-2361(94)90222-4.
Li X, Miao W, Lv Y, Wang Y, Gao C, Jiang D. TGA-FTIR investigation on the co-combustion characteristics of heavy oil fly ash and municipal sewage sludge. Thermochim Acta. 2018;666:1–9. https://doi.org/10.1016/j.tca.2018.05.023.
Kuan Y-H, Wu F-H, Chen G-B, Lin H-T, Lin T-H. Study of the combustion characteristics of sewage sludge pyrolysis oil, heavy fuel oil, and their blends. Energy. 2020. https://doi.org/10.1016/j.energy.2020.117559.
Khateeb AA, Elbaz AM, Guida P, Roberts WL. Influence of asphaltene concentration on the combustion of a heavy fuel oil droplet. Energy Fuels. 2018;32(12):12981–91. https://doi.org/10.1021/acs.energyfuels.8b03260.
Elbaz AM, Gani A, Hourani N, Emwas A-H, Sarathy SM, Roberts WL. TG/DTG, FT-ICR mass spectrometry, and NMR spectroscopy study of heavy fuel oil. Energy Fuels. 2015;29(12):7825–35. https://doi.org/10.1021/acs.energyfuels.5b01739.
Ciajolo A, Barbella R. Pyrolysis and oxidation of heavy fuel oils and their fractions in a thermogravimetric apparatus. Fuel. 1984;63(5):657–61. https://doi.org/10.1016/0016-2361(84)90162-5.
Ghashghaee M, Shirvani S. Two-step thermal cracking of an extra-heavy fuel oil: experimental evaluation, characterization, and kinetics. Ind Eng Chem Res. 2018;57(22):7421–30. https://doi.org/10.1021/acs.iecr.8b00819.
Nie F, Li Y, Tong K, Wu B, Zhang M, Ren W, et al. Volatile evolution during thermal treatment of oily sludge from a petroleum refinery wastewater treatment plant: TGA-MS, Py-GC (EGA)/MS and kinetics study. Fuel. 2020;278: 118332. https://doi.org/10.1016/j.fuel.2020.118332.
Menares T, Herrera J, Romero R, Osorio P, Arteaga-Pérez LE. Waste tires pyrolysis kinetics and reaction mechanisms explained by TGA and Py-GC/MS under kinetically-controlled regime. Waste Manage. 2020;102:21–9. https://doi.org/10.1016/j.wasman.2019.10.027.
Janković B, Manić N, Radović I, Janković M, Rajačić M. Model-free and model-based kinetics of the combustion process of low rank coals with high ash contents using TGA-DTG-DTA-MS and FTIR techniques. Thermochim Acta. 2019;679: 178337. https://doi.org/10.1016/j.tca.2019.178337.
Lin Y, Liao Y, Yu Z, Fang S, Lin Y, Fan Y, et al. Co-pyrolysis kinetics of sewage sludge and oil shale thermal decomposition using TGA–FTIR analysis. Energy Convers Manage. 2016;118:345–52. https://doi.org/10.1016/j.enconman.2016.04.004.
Sait HH, Hussain A, Salema AA, Ani FN. Pyrolysis and combustion kinetics of date palm biomass using thermogravimetric analysis. Bioresour Technol. 2012;118:382–9. https://doi.org/10.1016/j.biortech.2012.04.081.
Tahir MH, Cheng X, Irfan RM, Ashraf R, Zhang Y. Comparative chemical analysis of pyrolyzed bio oil using online TGA-FTIR and GC-MS. J Anal Appl Pyrolysis. 2020;150: 104890. https://doi.org/10.1016/j.jaap.2020.104890.
Lazdovica K, Kampars V, Grabis J. Effect of zinc-containing nanopowders on the catalytic intermediate pyrolysis of buckwheat straw by using TGA-FTIR method. J Anal Appl Pyrolysis. 2020;152: 104882. https://doi.org/10.1016/j.jaap.2020.104882.
Gou X, Zhao X, Singh S, Qiao D. Tri-pyrolysis: a thermo-kinetic characterisation of polyethylene, cornstalk, and anthracite coal using TGA-FTIR analysis. Fuel. 2019;252:393–402. https://doi.org/10.1016/j.fuel.2019.03.143.
Xiao R, Yang W, Cong X, Dong K, Xu J, Wang D, et al. Thermogravimetric analysis and reaction kinetics of lignocellulosic biomass pyrolysis. Energy. 2020. https://doi.org/10.1016/j.energy.2020.117537.
Rego F, Dias APS, Casquilho M, Rosa FC, Rodrigues A. Pyrolysis kinetics of short rotation coppice poplar biomass. Energy. 2020;207: 118191. https://doi.org/10.1016/j.energy.2020.118191.
Zhao S, Pu W, Varfolomeev MA, Liu Y, Liu Z. Oxidation characteristics of heavy oil and its SARA fractions during combustion using TG-FTIR. J Petrol Sci Eng. 2020;192: 107331. https://doi.org/10.1016/j.petrol.2020.107331.
Gabbott P. Principles and applications of thermal analysis. John Wiley & Sons; (2008).
Su S, Pohl JH, Holcombe D, Hart J. Techniques to determine ignition, flame stability and burnout of blended coals in pf power station boilers. Prog Energy Combust Sci. 2001;27(1):75–98. https://doi.org/10.1016/S0360-1285(00)00006-X.
Liu X, Chen M, Wei Y. Kinetics based on two-stage scheme for co-combustion of herbaceous biomass and bituminous coal. Fuel. 2015;143:577–85. https://doi.org/10.1016/j.fuel.2014.11.085.
Zhang K, Zhang K, Cao Y, Pan W-P. Co-combustion characteristics and blending optimization of tobacco stem and high-sulfur bituminous coal based on thermogravimetric and mass spectrometry analyses. Bioresour Technol. 2013;131:325–32. https://doi.org/10.1016/j.biortech.2012.12.163.
Li X-G, Ma B-G, Xu L, Hu Z-W, Wang X-G. Thermogravimetric analysis of the co-combustion of the blends with high ash coal and waste tyres. Thermochim Acta. 2006;441(1):79–83. https://doi.org/10.1016/j.tca.2005.11.044.
Li X, Lv Y, Ma B, Jian S, Tan H. Thermogravimetric investigation on co-combustion characteristics of tobacco residue and high-ash anthracite coal. Bioresour Technol. 2011;102(20):9783–7. https://doi.org/10.1016/j.biortech.2011.07.117.
Ma B-G, Li X-G, Xu L, Wang K, Wang X-G. Investigation on catalyzed combustion of high ash coal by thermogravimetric analysis. Thermochim Acta. 2006;445(1):19–22. https://doi.org/10.1016/j.tca.2006.03.021.
Niu S, Chen M, Li Y, Xue F. Evaluation on the oxy-fuel combustion behavior of dried sewage sludge. Fuel. 2016;178:129–38. https://doi.org/10.1016/j.fuel.2016.03.053.
Yang Z, Zhang S, Liu L, Li X, Chen H, Yang H, et al. Combustion behaviours of tobacco stem in a thermogravimetric analyser and a pilot-scale fluidized bed reactor. Bioresour Technol. 2012;110:595–602. https://doi.org/10.1016/j.biortech.2011.12.119.
Ordonez-Loza J, Chejne F, Jameel AGA, Telalovic S, Arrieta AA, Sarathy SM. An investigation into the pyrolysis and oxidation of bio-oil from sugarcane bagasse: kinetics and evolved gases using TGA-FTIR. J Environ Chem Eng. 2021;9(5): 106144. https://doi.org/10.1016/j.jece.2021.106144.
Chen G-B, Li J-W, Lin H-T, Wu F-H, Chao Y-C. A study of the production and combustion characteristics of pyrolytic oil from sewage sludge using the taguchi method. Energies. 2018;11(9):2260. https://doi.org/10.3390/en11092260.
Jayaraman K, Kök MV, Gökalp I. Combustion mechanism and model free kinetics of different origin coal samples: thermal analysis approach. Energy. 2020;204: 117905. https://doi.org/10.1016/j.energy.2020.117905.
Santos RGD, Loh W, Bannwart A, Trevisan O. An overview of heavy oil properties and its recovery and transportation methods. Braz J Chem Eng. 2014;31(3):571–90. https://doi.org/10.1590/0104-6632.20140313s00001853.
Poutsma ML. Fundamental reactions of free radicals relevant to pyrolysis reactions. J Anal Appl Pyrolysis. 2000;54(1–2):5–35. https://doi.org/10.1016/S0165-2370(99)00083-2.
Gautam R, Varma AK, Vinu R. Apparent kinetics of fast pyrolysis of four different microalgae and product analyses using pyrolysis-FTIR and pyrolysis-GC/MS. Energy Fuels. 2017;31(11):12339–49. https://doi.org/10.1021/acs.energyfuels.7b02520.
Gautam R, Vinu R. Unraveling the interactions in fast co-pyrolysis of microalgae model compounds via pyrolysis-GC/MS and pyrolysis-FTIR techniques. React Chem Eng. 2019;4(2):278–97. https://doi.org/10.1039/C8RE00227D.
Zervas E. Formation of oxygenated compounds (aldehydes, alcohols, organic acids) from propane flames. Environ Eng Sci. 2005;22(5):651–9. https://doi.org/10.1089/ees.2005.22.651.
Candeli A, Morozzi G, Zoccolillo L. Effect of fuel composition on the emission of phenols in the exhaust gas from a European car. Zentralbl Bakteriol Orig B. 1977;164(4):303–13.
Shin S, Im SI, Nho NS, Lee KB. Kinetic analysis using thermogravimetric analysis for nonisothermal pyrolysis of vacuum residue. J Therm Anal Calorim. 2016;126(2):933–41. https://doi.org/10.1007/s10973-016-5568-6.
Xu Z, Xiao X, Fang P, Ye L, Huang J, Wu H, et al. Comparison of combustion and pyrolysis behavior of the peanut shells in air and N2: kinetics, thermodynamics and gas emissions. Sustainability. 2020;12(2):464. https://doi.org/10.3390/su12020464.
Coats AW, Redfern J. Kinetic parameters from thermogravimetric data. Nature. 1964;201(4914):68–9. https://doi.org/10.1038/201068a0.
Huang S, Jia H, Sheng JJ. Research on oxidation kinetics of tight oil from Wolfcamp field. Pet Sci Technol. 2016;34(10):903–10. https://doi.org/10.1080/10916466.2016.1174714.
Huang S, Sheng JJ. An innovative method to build a comprehensive kinetic model for air injection using TGA/DSC experiments. Fuel. 2017;210:98–106. https://doi.org/10.1016/j.fuel.2017.08.048.
Zhao S, Pu W, Varfolomeev MA, Yuan C, Pan J, Wang R, et al. Low-temperature oxidation of light and heavy oils via thermal analysis: kinetic analysis and temperature zone division. J Petrol Sci Eng. 2018;168:246–55. https://doi.org/10.1016/j.petrol.2018.05.031.
Khansari Z, Kapadia P, Mahinpey N, Gates ID. A new reaction model for low temperature oxidation of heavy oil: experiments and numerical modeling. Energy. 2014;64:419–28. https://doi.org/10.1016/j.energy.2013.11.024.
Tran L-S, Wullenkord J, Li Y, Herbinet O, Zeng M, Qi F, et al. Probing the low-temperature chemistry of di-n-butyl ether: detection of previously unobserved intermediates. Combust Flame. 2019;210:9–24. https://doi.org/10.1016/j.combustflame.2019.08.022.
AlAbbad M, Badra J, Djebbi K, Farooq A. Ignition delay measurements of a low-octane gasoline blend, designed for gasoline compression ignition (GCI) engines. Proc Combust Inst. 2019;37(1):171–8. https://doi.org/10.1016/j.proci.2018.05.097.
Alabbad M, Li Y, AlJohani K, Kenny G, Hakimov K, Al-lehaibi M, et al. Ignition delay time measurements of diesel and gasoline blends. Combust Flame. 2020;222:460–75. https://doi.org/10.1016/j.combustflame.2020.09.008.
Javed T, Ahmed A, Lovisotto L, Issayev G, Badra J, Sarathy SM, et al. Ignition studies of two low-octane gasolines. Combust Flame. 2017;185:152–9.
Lee C, Ahmed A, Nasir EF, Badra J, Kalghatgi G, Sarathy SM, et al. Autoignition characteristics of oxygenated gasolines. Combust Flame. 2017;186:114–28.
Sarathy SM, Farooq A, Kalghatgi GT. Recent progress in gasoline surrogate fuels. Prog Energy Combust Sci. 2018;65:67–108. https://doi.org/10.1016/j.pecs.2017.09.004.
Sarathy SM, Kukkadapu G, Mehl M, Wang W, Javed T, Park S, et al. Ignition of alkane-rich FACE gasoline fuels and their surrogate mixtures. Proc Combust Inst. 2015;35(1):249–57.
Alturaifi SA, Rebagay RL, Mathieu O, Guo B, Petersen EL. A shock-tube autoignition study of jet, rocket, and diesel fuels. Energy Fuels. 2019;33(3):2516–25. https://doi.org/10.1021/acs.energyfuels.8b04290.
Burden S, Tekawade A, Oehlschlaeger MA. Ignition delay times for jet and diesel fuels: Constant volume spray and gas-phase shock tube measurements. Fuel. 2018;219:312–9. https://doi.org/10.1016/j.fuel.2018.01.113.
Gowdagiri S, Oehlschlaeger MA. Global reduced model for conventional and alternative jet and diesel fuel autoignition. Energy Fuels. 2014;28(4):2795–801. https://doi.org/10.1021/ef500346m.
Gowdagiri S, Wang W, Oehlschlaeger MA. A shock tube ignition delay study of conventional diesel fuel and hydroprocessed renewable diesel fuel from algal oil. Fuel. 2014;128:21–9. https://doi.org/10.1016/j.fuel.2014.02.064.
Yu L, Wang S, Wang W, Qiu Y, Qian Y, Mao Y, et al. Exploration of chemical composition effects on the autoignition of two commercial diesels: rapid compression machine experiments and model simulation. Combust Flame. 2019;204:204–19.
Wang H, Oehlschlaeger MA. Autoignition studies of conventional and Fischer-Tropsch jet fuels. Fuel. 2012;98:249–58. https://doi.org/10.1016/j.fuel.2012.03.041.
Allen C, Valco D, Toulson E, Edwards T, Lee T. Ignition behavior and surrogate modeling of JP-8 and of camelina and tallow hydrotreated renewable jet fuels at low temperatures. Combust Flame. 2013;160(2):232–9. https://doi.org/10.1016/j.combustflame.2012.10.008.
Friedman HL. New methods for evaluating kinetic parameters from thermal analysis data. J Polym Sci, Part B: Polym Lett. 1969;7(1):41–6. https://doi.org/10.1002/pol.1969.110070109.
Doyle C. Estimating isothermal life from thermogravimetric data. J Appl Polym Sci. 1962;6(24):639–42. https://doi.org/10.1002/app.1962.070062406.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6. https://doi.org/10.1246/bcsj.38.1881.
Trans AT. Joint convention of four electrical institutes. Res Rep Chiba Inst Technol. 1971;16:22–31.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404(1):163–76. https://doi.org/10.1016/S0040-6031(03)00144-8.
Sánchez-Jiménez PE, Pérez-Maqueda LA, Perejón A, Criado JM. Limitations of model-fitting methods for kinetic analysis: polystyrene thermal degradation. Resour Conserv Recycl. 2013;74:75–81. https://doi.org/10.1016/j.resconrec.2013.02.014.
Mian I, Li X, Jian Y, Dacres OD, Zhong M, Liu J, et al. Kinetic study of biomass pellet pyrolysis by using distributed activation energy model and Coats Redfern methods and their comparison. Bioresour Technol. 2019;294: 122099. https://doi.org/10.1016/j.biortech.2019.122099.
Song F, Wang X, Li T, Zhang J, Bai Y, Xing B, et al. Spectroscopic analyses combined with Gaussian and Coats-Redfern models to investigate the characteristics and pyrolysis kinetics of sugarcane residue-derived biochars. J Clean Prod. 2019;237: 117855. https://doi.org/10.1016/j.jclepro.2019.117855.
Sattar H, Muzaffar I, Munir S. Thermal and kinetic study of rice husk, corn cobs, peanut crust and Khushab coal under inert (N2) and oxidative (dry air) atmospheres. Renew Energy. 2020;149:794–805. https://doi.org/10.1016/j.renene.2019.12.020.
Acknowledgements
This work was supported by Air Products and the KAUST Clean Combustion Research Center. Facilities in the KAUST Analytical Core Labs were used in this work. Also, the authors acknowledge Dr. Younes Mourad of Saudi Aramco for his help in providing the oil samples.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix A
Elemental analysis
The elemental (CHNS) analysis of the HFO and VRO was performed in a Thermo Flash 2000 (Thermo Fisher Scientific, USA) organic elemental analyzer. In a typical analysis, 2.5–3 mg of sample was used. The combustion tube present in the analyzer was heated to around 1000 °C in the presence of air. To determine carbon, hydrogen, nitrogen, and sulfur content in the sample, the elements were converted to their respective oxides. Gas chromatography/thermal conductivity detector (GC/TCD) was used to separate and detect these oxides evolving from the combustion of the samples. The GC/TCD is pre-calibrated for the determination of carbon, hydrogen, nitrogen, and sulfur content. It can be noted that the nitrogen oxides were reduced to N2 via Cu-based catalyst before the identification in the GC/TCD. For the determination of oxygen, a different experiment was conducted on the same equipment using a nickel carbon catalyst in the combustion tube. The oxygen present in the evolved vapors is converted to carbon monoxide in the presence of the catalyst. The GC/TCD is pre-calibrated to determine the oxygen based on the amount of carbon monoxide.
SARA methodology
SARA fractionation was achieved by following a modified ASTM D4124 method. The ASTM D4124 is modified by replacing the chromatography with DCVC to allow the separation of larger sample loads, compared with the ASTM method, achieving a fast separation with low solvent consumption and without compromising the resolution between fractions. First, around 10 g of each oil was treated with 400 mL of n-heptane in a reflux setup at 98˚C for three hours. The collected mixture was kept to settle for 24 h, and then solids were separated by filtration (Whatman grade 42) and washed with additional n-heptane until the eluent was clear. The solids were recovered by hot toluene and rota-evaporated to yield asphaltenes, which were quantified gravimetrically. The supernatant, maltenes, was purified by removing the excess solvent and then subjected to dry column vacuum chromatography (DCVC) using a glass column (Chemglass 41 × 305 mm) packed with alumina (Mesh 80–200) as a sorbent to yield individual saturates, aromatics, and resins fractions. All the fractions were quantified gravimetrically once the excess solvent was removed by rota-evaporation. The same eluotropic series of the ASTM method was used. The overall recovery for HFO and VRO was found to be around 98%. Figure
20 illustrates the SARA procedure and shows a photograph of the fractions collected.
Kinetic analysis
The apparent activation energy changes with the extent of the reaction owing to the multi-step mechanism assumed by the oils during pyrolysis and combustion. The variation of apparent activation energy with the conversion can be given by isoconversional methods. The use of isoconversional models results in the deconvolution of the complex procedure to perform kinetic analysis of these multi-step processes of these feedstock. The utilization of these models assumes that the thermal decomposition of heavy oils occurs as follows:
The conversion of oil can be described by a general equation expressed as:
T,\(\beta\), Ea, R, A, and f(α) represent temperature (in K), heating rate (in °C min−1 or K min−1), apparent activation energy (in kJ mol−1), universal gas constant (in J mol−1 K−1), pre-exponential factor (min−1), and functional dependence of rate on the extent of conversion, respectively. Here α is the extent of conversion normalized with respect to the residual mass of the sample observed in the TGA and is given as follows:
mi, mt, and m∞ represent the initial sample mass, mass of the sample at any instant ‘t,’ and steady-state mass (residual mass). Rearranging and integrating Eq. (A2) results in the following expression:
It is assumed for the isoconversional models that the reaction rate for a particular conversion depends only on temperature. The multi-step mechanism involved in the thermal decomposition of organics is identified by the variation of apparent activation energy with conversion obtained from various models. Taking natural logarithm both sides of Eq. (A2) and rearranging gives:
Equation (A4) is known as Freidman method, which is a differential isoconversional method [67]. Thermogravimetric mass loss data were utilized for evaluation of \(\frac{\text{d}\alpha }{\rm{d}T}\). For the same conversion across different heating rates, \(\mathrm{ln}\beta \left(\frac{\rm{d}\alpha }{\text{d}T}\right)\) vs 1/T data were plotted. To the plotted co-ordinates, straight lines were fitted, and the slopes of these lines \(\left(\frac{-{E}_{\mathrm{a}}}{R}\right)\) were utilized to determine the apparent activation energies for different conversions. Multi-step mechanism involved during pyrolysis and combustion is understood by variation of apparent activation energy with conversion.
It can be noted that the TG analysis results in integral data, and differentiating integral data magnifies the noise. So, there can be errors in the derivative of conversion with respect to temperature. Hence the choice of integral methods is justified over differential methods.
Substituting Ea/RT as x in Eq. (A4), which is also known as temperature integral, the following expression is obtained:
Different temperature integrals result from the different mechanisms involved during the thermal decomposition of oils. Flynn–Wall–Ozawa (FWO) method assumes the change in the apparent activation energy to be constant during the thermal decomposition and employs Doyle’s approximation [68] in order to have a solution of the temperature integral.
Taking natural logarithm both sides and rearranging Eq. (A6), the mathematical form of FWO method is obtained [69]:
At different heating rates, for the same conversion, \({\text{ln}} \beta\) vs. 1/T data were plotted. Different straight lines were plotted for multiple conversions, and \(\frac{-1.052{E}_{\text{a}}}{R}\) was used to determine the variation of apparent activation energy with conversion. Applying one more Doyle’s approximation for having the solution of the temperature integral, Eqs. (A9) and (A10) represent the approximation used and the mathematical form of the Kissinger–Akahira–Sunose (KAS) method, respectively [70].
The slope of the straight line \(\left(\frac{-{E}_{\mathrm{a}}}{R}\right)\) fitted to the \(\mathrm{ln}\frac{\beta }{{T}^{2}}\) vs 1/T obtained for the same conversion at different heating rates is used to determine the apparent activation energy at a particular conversion. Following to this, the approximation proposed by Starinks [48, 71] to Eq. (A10) allows more accurate estimation of apparent activation energy. The mathematical expression of Starink’s method is as follows:
The procedure of the determination of apparent activation energies is same as for the previous methods, i.e., slope of the straight lines plotted for multiple conversions was utilized.
For the oxygen-rich case, a model-fitting method was also used along with model-free methods. The apparent kinetics of thermal decomposition of VRO was studied using Coats and Redfern, and the mathematical expression is as follows [48]:
It can be noted that this method was not used for the evaluation of apparent activation energies of thermal decomposition of both the oils under different cases which are studied. This model was used for the oxygen-rich case where the model-free methods are inapplicable to determine the apparent activation energies during the oxidation of both the oils. The limitations associated are as follows: First, this method, owing to the assumptions, may not describe the real thermal decomposition process [72]. Second, the method is not capable of describing the reactions with low activation energies associated with low temperatures (< 200 °C) [48, 72]. Due to the simplicity associated with the model for the evaluation of apparent kinetic parameters utilizing single heating rates, it is widely used in recent studies involving TGA of various biomasses under various atmospheres [73,74,75]. In Equation (A12), \(g(\alpha )\), functional dependence on conversion, depends on the reaction mechanism assumed by the oil during thermal decomposition.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
AlAbbad, M., Gautam, R., Romero, E.G. et al. TG-DSC and TG-FTIR analysis of heavy fuel oil and vacuum residual oil pyrolysis and combustion: characterization, kinetics, and evolved gas analysis. J Therm Anal Calorim 148, 1875–1898 (2023). https://doi.org/10.1007/s10973-022-11871-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11871-x