Abstract
Thermally stimulated discharge current (TSD) tests on PVC, chlorinated polyethylene, chlorinated PVC, and chlorinated paraffins always show a relatively low-intensity transition in the − 100 to − 80 °C range. Several chlorine-containing polymers and one chlorinated paraffin were tested up to 70% chlorine content by short-circuited TSD method. The intensities of these transitions show a maximum at about 40% chlorine content except for PVC. Knowing the random nature of chlorination, it is assumed that this transition origins from the rotational movement of neighboring –CH2–CHCl– monomeric units.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chlorine-containing polymers are widely used in everyday life. The best known of them is polyvinyl chloride (PVC), the third most important commodity polymer. Chlorinated polyethylene (CPE) is used as impact modifier, flame retardant additive and in paints. Chlorinated PVC (CPVC) has high glass transition temperature and has an important role in hot-water piping. Polychloroprene (Neoprene) is an important synthetic rubber. Chlorinated paraffins (CP) are used as secondary plasticizers and flame retardants. The chlorine-containing polymers are thoroughly investigated; most of their relaxation processes are known. Some of them, in particular the randomly chlorinated polymers (CPE, CPVC), have multiple transitions. Hedvig [1] demonstrated α and β relaxation of PVC; these are detectable by dynamic mechanical analysis (DMA), AC dielectric and TSD methods as well. Despite of the high polarity of the –C–Cl bond, few references can be found on the low-temperature peak of chlorine-containing polymers. Galop [2] used short-circuit TSD for testing polymers and stated that the frozen-in stresses also produce depolarization current. Interestingly, a low-temperature peak was found in polypropylene at − 50 °C and explained as “relaxation of lateral groups.” del Val [3] used the analogous mechanical method, the thermally stimulated creep (TSC) for testing PVC and PVC-CPE blends. Deformation rate curves were resolved by windowing technique and found low-temperature transitions at about − 80 °C for all tested polymers. This transition was assigned to β relaxation. However, β relaxation of PVC is in the range of − 30 °C [4]. Guarrotxena [5] used the TSD technique for chlorinated PVC. This material contains less –CH2–CHCl– monomer units because of the chlorination of the neighboring carbon atoms, while the frequency of –CHCl– units increases. However, the depolarization processes below 0 °C were not investigated.
Kollár and Zsoldos [6] tested PVC-PE blends by different thermal methods but over − 80 °C and observed the β relaxation of PVC only. Mahrous [7] investigated CPVC-polyester blends by TSD but over room temperature only.
Nogales [8] used combined dielectric techniques (TSD and A.C. dielectric) for investigation of molecular motions in PVC without deeply analyzing the low temperature region.
Several TSD measurements on chlorine-containing polymers revealed that there is peak of low intensity in the − 100 to − 75 °C range and its origin is not known [9].
Experimental
Materials
Chlorinated polyethylene samples up to 33.8% chlorine content were taken from the BorsodChem’s CPE plant reactors. During this process, additive-free high-density polyethylene powder is chlorinated in aqueous slurry. To avoid the blocking of material the reaction temperature must be kept below the melting temperature of PE, while the Cl content is less than 17–19%. Thereafter, the temperature is increased. Samples were taken from the reactor then washed acid free and dried. CPE-36, 40 and 48 were also industrial products from the same raw material. CPE-53 and 60 are experimental grades produced in pilot plant reactors.
PVC sample was a commercial suspension type of 58 K-value. CPVC a low molecular weight (injection molding grade) type of 68% Cl content.
The 70% chlorine-containing material was commercial-type solid chlorinated paraffin (CP) intended for use as flame retardant.
Sample preparation
All polymer samples were prepared by roll milling on a Schwabenthan 150U electrically heated laboratory roll-mill at 20/24 rpm (front/back). 160 °C was used for CPE, 175 °C for PVC and 190 °C for CPVC. CPE was stabilized with 1 phr calcium stearate. PVC and CPVC samples were stabilized with 1.5 phr organotin stabilizer. The different rheological behaviors made necessary the different processing temperatures. CPVC, e.g., cannot melt at the optimal rolling temperature of CPE.
Roll-milled sheets of about 0.5–1 mm thickness were compression molded over the roll milling temperature by 5 °C. The actual thickness was used in the evaluation program. Disks were die cut, and gold electrodes were sputter coated. The thickness of gold electrodes was about 8–10 nm. The chlorinated paraffin was soaked onto borosilicate filter paper (SIGMA F7769; 2.6 μm, relative pore volume 87 ± 2%.). The method was described in detail in the previous paper [10].
Instruments
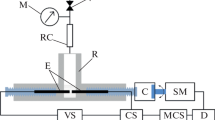
The TSD measurements were carried out with the TSD part of Multirelax instrument constructed by Műanyag-ipari Kutató Intézet (Research Institute of Plastics, Budapest) described in [11, 12]. Polarizing supply was designed and assembled by the author.
Summarized technical data of the instrument:
Electrometer sensitivity: 0.1 pA to 1 mA.
Temperature range: − 150 to 250 °C.
Sample size:53 mm diameter,
0.05–1 mm thickness.
Electrode size: 38 mm diameter (11.3 cm2).
Heat transfer:metal (brass) block.
Cooling:liquid nitrogen.
Cooling and heating rate: 1 K min−1 (fixed).
Polarizing voltage: 60 V to 3 kV.
Actual polarizing voltage:1200 V.
Dynamic mechanical measurement (demonstrated in Fig. 1.) was performed with a Rheometric Scientific DMTA MK-III instrument in dual cantilever mode at 1 Hz frequency and 64 µm p–p amplitude equivalent to about 0.1% deformation. Heating rate was 2 K min−1in the − 120 to 100 °C range.
Brief description of the experimental technique and evaluation
The usual representation of a TSD measurement is a function of current against temperature. For the sake of simpler comparison normalization of the measured current is suggested, i.e., transforming the raw curves to equal electrode area and electric field. In this paper, all results are transformed to a field of 1 kVmm−1 and to a 30 cm2 electrode area as described in [4, 10].
The evaluation program was compiled by the author.
In the case of short circuit TSD during depolarization, the external field is zero, the obtained current is the algebraic sum of the currents originating from the overlapping processes.
The energies of activation values were determined from the half-width of the peaks as described in [11]. Individual transitions are calculated by Eq. (13) in the same paper.
The Δε relaxation strength represents the permittivity change during the depolarization:
E is the polarizing field, σ is the surface charge density, and ε0 is the permittivity of vacuum:
where \(\mathcal{A}\) is the electrode area; Q is the full charge of a transition and can be determined by integration of current by time.
Results and discussion
Because of the stepwise chlorination of polyethylene, the CPE always consists of two structural unique units. One of them has higher, the other lower chlorine content than the average as it was demonstrated by a swelling procedure in a former paper [13]. However, dynamic mechanical method (DMA) shows a single broad transition only (Fig. 1), while the TSD reveals these phases and gives well-separated peaks as shown in Fig. 2. The high resolving power of TSD makes the analysis of the low temperature region possible. The DMA and the main transitions are not the target of this paper.
All chlorine-containing materials tested show a weak transition in the − 120… − 70 °C range. This transition is demonstrated in Fig. 2 magnified 25 times. The data of results on all tested materials are summarized in Table 1.
Energy of activation values is relatively low, and the scattering of the values is considerable as shown in Fig. 3. Plotting the current maxima of normalized currents as function of chlorine content in Fig. 4 shows a bimodal curve. Plotting the relaxation strength (∆ε) in Fig. 5, the result is similar. The first maximum occurs at about 10–12% by mass, and the second one at about 40% by mass in both representations. This peak maximum is well below the chlorine content of PVC (56.7%).
It is assumed that this low-temperature transition in Cl containing polymers is caused by rotational motion of –CH2–CHCl– triads as shown in Fig. 6. In regular PVC structure such structural units are along the backbone. However, the substitution reaction, thus the formation of these triads, is also a statistical process using any kind of chlorination. Increasing the chlorine content, the probability of these units is increasing, but the probability of defective structures is also increasing in parallel. At about 40% Cl content, the previously created regular structures are destroyed. Chlorination of PVC leads to decreasing the number of existing regular triads. Therefore, in case of CPE over 40% Cl content, the intensity of this transition decreases. It is interesting that in case of the chlorinated paraffin of 70% Cl content, this peak is still detectable, although it is near to the noise-limited sensitivity of the instrument used.
The first maximum observed at 10–12% chlorine in both relaxation strength (Δε) and Imax can be explained by chlorination technology. As it was mentioned, the temperature in first stage of chlorination is below the melting range of polyethylene. At this temperature, the crystalline part of the polyethylene remains intact; the reaction takes place in the amorphous phase only. Because the amorphous phase of HDPE is about 30% at 10–12% Cl content, the number of regular units starts to decrease. Melting the crystalline part by increasing the temperature the full amount of polyethylene becomes able for chlorination.
TSD method showed undoubtedly the presence of the transition and proved excellent tool for observing these processes of low intensity. The results support the concept about the structure formation during chlorine substitution reaction.
Abbreviations
- PVC:
-
Poly(vinyl chloride)
- CPVC:
-
Chlorinated PVC
- CPE:
-
Chlorinated polyethylene
- CP:
-
Chlorinated paraffin
- DMA:
-
Dynamic mechanical analysis
- TSD:
-
Thermally stimulated discharge
- A :
-
Energy of activation (J mol−1; kJ mol−1; eV/molecule)
- \(\mathcal{A}\) :
-
Area (m2; cm2)
- E :
-
Electric field (Vm−1; kV mm−1)
- I :
-
Current (in general) (A)
- I d :
-
Depolarization current (pA)
- I m :
-
Peak current (pA)
- Q :
-
Electric charge (As; pAs)
- T :
-
Absolute temperature (K)
- R :
-
Gas constant (8.314 J mol−1 K−1; 5.189·10–19 eV mol−1 K−1.)
- ε 0 :
-
Dielectric constant of vacuum (8.854·10–12 F m−1)
- Δε :
-
Relaxation strength (εr0−εr∞) here (σ/Eε0)
- σ :
-
Charge density (As m−2)
- τ :
-
Relaxation time (s)
- τ 0 :
-
Preexponential factor (s)
- ϑ :
-
Temperature in centigrades (°C)
References
Hedvig P. Dielectric spectroscopy of polymers. Bristol: Hilger; 1977.
Galop M. Characterization of polymers using the thermally stimulated current technique. J Therm Anal. 1997. https://doi.org/10.1007/BF01987427.
del Val JJ, Lacabanne C, Hilther A. A thermostimulated creep study of blends of poly(vinyl chloride) and chlorinated polyethylene. J Appl Phys. 1988. https://doi.org/10.1063/1.340395.
Marossy K. Assessment of activation energies of transitions by thermally stimulated discharge current (TSD) experiments. Polym Bull. 1989. https://doi.org/10.1007/BF00255214.
Guarrotxena N, Contreas J, Martinez G, Millán J. Effect of [CHCl]n≥3 structures on the origin and relaxation of dipolar and trapped electric space charges in chlorinated PVC. Polym Bull. 1998. https://doi.org/10.1007/s002890050374.
Kollár M, Zsoldos G. Investigating poly-(vinyl-chloride)-polyethylene blends by thermal methods. J Therm Anal Calorim. 2012. https://doi.org/10.1007/s10973-011-1939-1.
Mahrous S. Study of dielectric relaxation of chlorinated polyvinyl chloride (CPVC)-poly(ethylene succinate) (PES) blend. Int J Polym Mater. 1999. https://doi.org/10.1080/00914039908012141.
Nogales A, Sauer BB. Cooperative motions in PVC studied by thermally stimulated currents: comparison with A.C. dielectric derivative analysis. J Polym Sci Part B Polym Phys. 1988. https://doi.org/10.1002/(sici)1099-0488(19980415)36:53.0.co;2-c.
Mahrous SJ. Thermally stimulated current study of relaxation in PVC stabilized with zinc carboxilate. J Macromol Sci B. 1995;34(4):369–78. https://doi.org/10.1080/00222349508219499.
Tamási K, Marossy K. Combined thermal analysis of plant oils. J Therm Anal Calorim. 2021. https://doi.org/10.1007/s10973-020-10470-y.
Marossy K. Practical approach to thermally stimulated discharge (TSD) method on polymers. J Thermal Anal Calorim. 2017. https://doi.org/10.1007/s10973-017-6098-6.
Yalof SA, Hedvig P. Etma: the combined electrical, thermal, mechanical analysis of the behavior of materials with Unirelax. Thermochim Acta. 1976. https://doi.org/10.1016/0040-6031(76)80074-3.
Marossy K, Tóth J. Anomalous behaviour of PVC–CPVC–CPE blends. Plast Rubber Compos Proc Appl. 2005. https://doi.org/10.1179/174328905X66162.
Acknowledgments
The research was carried out as part of the EFOP-3.6.1-16-2016-00011 “Younger and Renewing University – Innovative Knowledge City—institutional development of the University of Miskolc aiming at intelligent specialisation” project implemented in the framework of the Széchenyi 2020 program. The realization of this project is supported by the European Union and co-financed by the European Social Fund.
Funding
Open access funding provided by University of Miskolc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marossy, K. Thermally stimulated discharge (TSD) study of low-temperature transitions in chlorine-containing polymers. J Therm Anal Calorim 148, 1899–1903 (2023). https://doi.org/10.1007/s10973-022-11803-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11803-9