Abstract
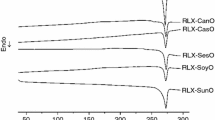
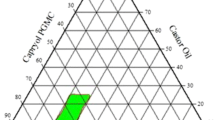
Testosterone is a cholesterol-derived steroidal hormone responsible for male sexual characteristics. Besides undergoing intense hepatic and intestinal metabolism, it presents low aqueous solubility and low intestinal permeability. Various strategies have been investigated to improve the bioavailability of poorly water-soluble such as testosterone, including the use of lipid systems. The main goal of this work was to carry out a pre-formulation study for the development a liquid self-emulsifying drug delivery system (L-SEDDS) with testosterone. The compatibility of the drug with oils and surfactants was investigated using DSC, TG, FTIR and isothermal stress test (IST). Interactions with testosterone were identified for oleic acid, Span 80 and Castor oil, in which greater changes were observed in the DSC and DTG thermal profiles. The incompatibility of the testosterone with these excipients was confirmed by the IST after 15 or 30 days of storage at 50 °C. A phase diagram was constructed using different proportions (w/w) of Captex 355, Tween 80 and Span 85. Formulations were characterized for size, PdI and zeta potential. F4 showed the highest zeta potential values (− 30.70 ± 2.28 mV and − 33.25 ± 1.77 mV) and lowest PdI values (0.232 ± 0.001 and 0.244 ± 0.011) (before and after drug incorporation, respectively). The pre-formulation studies enabled a rational selection of excipients for the development of L-SEDDS containing testosterone, thus reinforcing the importance and applicability of analytical and thermo-analytical techniques in the development of new formulations.




Similar content being viewed by others
References
Luetjens CM, Weinbauer GF. Testosterone: biosynthesis, transport, metabolism and (non-genomic) actions. In: Nieschlag E, Behre HM, editors. Testosterone action, deficiency, substitution. 4th ed. New York: Cambridge University Press; 2012. p. 1–591.
Hackett G, Kirby M, Edwards D, et al. British society for sexual medicine guidelines on adult testosterone deficiency, with statements for UK practice. J Sex Med. 2017;14:1504–23.
Okimoto K, Rajewski RA, Stella VJ. Release of testosterone from an osmotic pump tablet utilizing (SBE)(7m)-β-cyclodextrin as both a solubilizing and an osmotic pump agent. J Control Release. 1999;58(1):29–38.
Eixarch H, Haltner-Ukomadu E, Beisswenger C, Bock U. Drug delivery to the lung: Permeability and physicochemical characteristics of drugs as the basis for a pulmonary biopharmaceutical classification system (pBCS). J Epithel Biol Pharmacol. 2010;3:1–14.
Frey H, Aakvaag A, Saanum D, Falch J. Bioavailability of oral testosterone in males. Eur J Clin Pharmacol. 1979;16(5):345–9.
Täuber U, Schröder K, Düsterberg B, Matthes H. Absolute bioavailability of testosterone after oral administration of testosterone-undecanoate and testosterone. Eur J Drug Metab Pharmacokinet. 1986;11(2):145–9.
Daggett PR, Wheeler MJ, Nabarro JDN. Oral testosterone, a reappraisal. Horm Res. 1978;9:121–9.
Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6:231–48.
Dahan A, Hoffman A. Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J Control Release. 2008;129(1):1–10.
Serajuddin ATM. Salt formation to improve drug solubility. Adv Drug Deliv Rev. 2007;59(7):603–16.
Joshi JT. A review on micronization techniques. J Pharm Sci Technol. 2011;3(7):651–81.
Vandana KR, Prasanna Raju Y, Harini Chowdary V, Sushma M, Vijay KN. An overview on in situ micronization technique—an emerging novel concept in advanced drug delivery. Saudi Pharm J. 2014;22(4):283–9.
Cappello B, Di Maio C, Iervolino M, Miro A. Improvement of solubility and stability of valsartan by hydroxypropyl-β-cyclodextrin. J Incl Phenom Macrocycloc Chem. 2006;54(3–4):289–94.
Modi A, Tayade P. Enhancement of dissolution profile by solid dispersion (kneading) technique. AAPS PharmSciTech. 2006;7(3):1–6.
Dalvi PB, Gerange AB, Ingale PR. Solid dispersion: strategy to enhance solubility. J Drug Deliv Ther. 2015;5(2):20–8.
Venkatesh G, Majid MIA, Mansor SM, Nair NK, Croft SL, Navaratnam V. In vitro and in vivo evaluation of self-microemulsifying drug delivery system of buparvaquone. Drug Dev Ind Pharm. 2010;36(6):735–45.
Burra M, Jukanti R, Janga KY, et al. Enhanced intestinal absorption and bioavailability of raloxifene hydrochloride via lyophilized solid lipid nanoparticles. Adv Powder Technol. 2013;24(1):393–402.
Muchow M, Maincent P, Müller RH, Keck CM. Testosterone undecanoate—increase of oral bioavailability by nanostructured lipid carriers (NLC). J Pharm Technol Drug Res. 2013;2:1–10.
Hua Y, Li W, Cheng Z, et al. Solidification of nanostructured lipid carriers loaded testosterone undecanoate: in vivo and in vitro study. Drug Res. 2018;68:457–64.
Jo K, Kim H, Khadka P, et al. Enhanced intestinal lymphatic absorption of saquinavir through supersaturated self-microemulsifying drug delivery systems. Asian J Pharm Sci. 2019;16(58):1–11.
Zanchetta B, Chaud MV, Helena M, Santana A. SEDDS: Nanocarrier system for a new cardiac dysfunction drug. Eur J Biomed Pharm Sci. 2015;2(7):96–111.
Sharma S, Sharma AD, Naseer MA, Singh R. Formulation and evaluation of self emulsifying drug delivery system of ibuprofen using castor oil. Int J Pharm Pharm Sci. 2011;3(4):299–302.
Patil SL, Pharm IJ, Sci B, Nigade PM, Tiwari SS. Self emulsifying drug delivery system (SEDDS): a review. Int J Pharm Biol Sci. 2012;2(2):42–52.
Bharate SS, Bharate SB, Bajaj AN. Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: a comprehensive review. J Excipients Food Chem. 2010;1(3):3–26.
Silva LAD, Teixeira FV, Serpa RC, et al. Evaluation of carvedilol compatibility with lipid excipients for the development of lipid-based drug delivery systems. J Therm Anal Calorim. 2016;123(3):2337–44.
Veras KS, Fachel FNS, Pittol V, et al. Compatibility study of rosmarinic acid with excipients used in pharmaceutical solid dosage forms using thermal and non-thermal techniques. Saudi Pharm J. 2019;27(8):1138–45.
Ujhelyi Z, Vecsernyés M, Fehér P, et al. Physico-chemical characterization of self-emulsifying drug delivery systems. Drug Discov Today Technol 2018:81–86.
Borhade V, Pathak S, Sharma S, Patravale V. Clotrimazole nanoemulsion for malaria chemotherapy. Part I: preformulation studies, formulation design and physicochemical evaluation. Int J Pharm. 2012;431:138–48.
Li F, Hu R, Wang B, et al. Self-microemulsifying drug delivery system for improving the bioavailability of huperzine A by lymphatic uptake. Acta Pharm Sin B. 2017;7(3):353–60.
Swerdloff RS, Dudley RE. A new oral testosterone undecanoate therapy comes of age for the treatment of hypogonadal men. Ther Adv Urol. 2020;12:1–16.
Silva LAD, Cintra ER, Alonso ECP, et al. Selection of excipients for the development of carvedilol loaded lipid-based drug delivery systems. J Therm Anal Calorim. 2017;130(3):1593–604.
Teixeira FV, Alves GL, Ferreira MH, Taveira SF, da Cunha Filho MSS, Marreto RN. Preformulation studies to guide the development of raloxifene lipid-based delivery systems. J Therm Anal Calorim. 2018;132(1):365–71.
Kumar N, Goindi S, Saini B. Thermal characterization and compatibility studies of itraconazole and excipients for development of solid lipid nanoparticles. J Therm Anal Calorim. 2014;115:2375–83.
Khoo SM, Humberstone AJ, Porter CJh, Edwards GA, Charman WN. Formulation design and bioavailability assessment of lipidic self- emulsifying formulations of halofantrine. Int J Pharm. 1998;167(1–2):155–64.
Grove M, Anette M, Nielsen JL, Pedersen GP. Bioavailability of seocalcitol II : Development and characterisation of self-microemulsifying drug delivery systems ( SMEDDS ) for oral administration containing medium and long chain triglycerides. Eur J Pharm Sci. 2006;28:233–42.
Balata GF, Essa EA, Shamardl HA, Zaidan SH, Abourehab MAS. Self-emulsifying drug delivery systems as a tool to improve solubility and bioavailability of resveratrol. Drug Des Devel Ther. 2016;10:117–28.
Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–82.
Pouton CW, Porter CJH. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev. 2008;60(6):625–37.
Kalepu S, Manthina M, Padavala V. Oral lipid-based drug delivery systems—an overview. Acta Pharm Sin B. 2013;3(6):361–72.
Land LM, Li P, Bummer PM. The influence of water content of triglyceride oils on the solubility of steroids. Pharm Res. 2005;22(5):784–8.
Kalkura SN, Devanarayanan S. Crystal growth of steroids in silica gel: testosterone. J Cryst Growth. 1989;94(3):810–3.
Bhowmik BB, Sa B, Mukherjee A. Preparation and in vitro characterization of slow release testosterone nanocapsules in alginates. Acta Pharm. 2006;56:417–29.
Rowe RC, Sheskey PJ, Quinn ME. Handbook of pharmaceutical excipients. 2009.
Zhao H, Park DW, Kim SK, Lee CH, Kim DD. The effects of pressure-sensitive adhesives and solubilizers on the skin permeation of testosterone from a matrix-type transdermal delivery system. Drug Dev Ind Pharm. 2002;28(9):1125–31.
Faramarzi MA, Yazdi MT, Amini M, Mohseni FA. Microbial production of testosterone and testololactone in the culture of Aspergillus terreus. World J Microbiol Biotechnol. 2004;20:657–60.
Wasylaschuk WR, Harmon PA, Wagner G, et al. Evaluation of hydroperoxides in common pharmaceutical excipients. J Pharm Sci. 2007;96(1):106–16.
Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Inst Food Technol. 2006;5:169–86.
Qi B, Zhang Q, Sui X, Wang Z, Li Y, Jiang L. Differential scanning calorimetry study—assessing the influence of composition of vegetable oils on oxidation. Food Chem. 2016;194:601–7.
Dixit AR, Rajput SJ, Patel SG. Preparation and bioavailability assessment of SMEDDS containing valsartan. AAPS PharmSciTech. 2010;11(1):314–21.
Quan D, Gui-xia X, Xiang-gen W. Studies on preparation and absolute bioavailability of a self-emulsifying system containing Puerarin. Chem Pharm Bull. 2007;55(5):800–3.
Balakumar K, Raghavan CV, Selvan NT, Prasad RH, Abdu S. Self nanoemulsifying drug delivery system (SNEDDS) of Rosuvastatin calcium: Design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf B Biointerfaces. 2013;112:337–43.
Wei L, Sun P. Preparation and evaluation of SEDDS and SMEDDS containing carvedilol. Drug Dev Ind Pharm. 2005;31:785–94.
Agrawal AG, Kumar A, Gide PS. Colloids and Surfaces B : Biointerfaces Self emulsifying drug delivery system for enhanced solubility and dissolution of glipizide. Colloids Surf B Biointerfaces. 2015;126:553–60.
Pujara ND. Self-emulsifying drug delivery system: a novel approach. Int J Curr Pharm Res. 2012;4(2):18–23.
Jeevana JB, Sreelakshmi K. Design and evaluation of self-nanoemulsifying drug delivery system of flutamide. J Young Pharm. 2011;3(1):4–8.
Patel J, Patel A, Raval M, Sheth N. Formulation and development of a self-nanoemulsifying drug delivery system of irbesartan. J Adv Pharm Technol Res. 2011;2(1):1–8.
Acknowledgements
This work was financially supported by the following Brazilian research funding agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Pesquisas (FINEP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – Finance code 001).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Bárbara Cristina Campos Ribeiro and Emilio Ramos Cintra. The first draft of the manuscript was written by Luís Antônio Dantas Silva and Danielle Guimarães Almeida Diniz. Resources and funding supporte by Eliana Martins Lima. All authors commented on previous versions of the manuscript and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ribeiro, B.C.C., Cintra, E.R., Lima, E.M. et al. Evaluation of testosterone compatibility with different excipients for the development of a self-emulsifying drug delivery system. J Therm Anal Calorim 148, 159–168 (2023). https://doi.org/10.1007/s10973-022-11751-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11751-4