Abstract
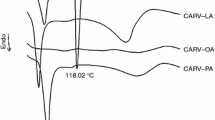
Carvedilol (CARV) is a widely used drug, which has shown low oral bioavailability. Lipid-based drug delivery systems (LBDDS) appear as promising alternatives to overcome CARV pharmacokinetic issues. Despite the pharmaceutical potential of LBDDS, the lipids used in these systems present stability issues and their use demands caution. This study set out to evaluate different lipid vehicles for CARV in terms of their ability to dissolve the drug and preserve its stability in LBDDS. The CARV solubility was determined in nine commonly used lipid excipients. Thermal and spectroscopic analysis (DSC, TG, FTIR), and accelerated stability studies were used to characterize drug-excipient interaction. CARV has highest solubility in polyethoxylated castor oil (PcasO), castor oil (CasO) and caprylic/capric triglycerides (CCT), ranging from 70 to 4 mg mL−1. DSC and FTIR analyses showed evidence of drug-oil interaction. TG results gained in inert and oxidative atmosphere showed that these interactions lead to a drug stability loss at temperatures above 300 °C, especially in binary mixtures of CARV with CCT, sesame oil (SesO) and CasO. On the other hand, in accelerated stability studies performed at 50 °C, CARV solutions in CCT and SesO were the most stable systems. The results of this study showed that CARV had high solubility and stability in PcasO. This means that PcasO is suitable for systems demanding high drug concentration and which are produced at high temperatures. On the other hand, SesO and CCT can give rise to less concentrated systems which can be stable when produced at low temperatures.







Similar content being viewed by others
References
Galanopoulou O, Rozou S, Antoniadou-Vyza E. HPLC analysis, isolation and identification of a new degradation product in carvedilol tablets. J Pharm Biomed Anal. 2008;48:70–7.
Qvigstad E, Sjaastad I, Bøkenes J, Schiander I, Solberg L, Sejersted OM, Osnes J-B, Skomedal T. Carvedilol blockade α1- and β-adrenoceptor induced inotropic responses in rats with congestive heart failure. Eur J Pharmacol. 2005;516:51–9.
Henderson LS, Tenero DM, Baidoo CA, Campanile AM, Harter AH, Boyle D, Danoff TM. Pharmacokinetic and pharmacodynamic comparison of controlled-release carvedilol and immediate-release carvedilol at steady state in patients with hypertension. Am J Cardiol. 2006;98:17L–26L.
Dantas EM, Pimentel EB, Andreão RV, Cichoni BS, Gonçalves CP, Zaniqueli DA, Baldo MP, Rodrigues SL, Mill JG. Carvedilol recovers normal blood pressure variability in rats with myocardial infarction. Auton Neurosci. 2013;177:231–6.
Hamed R, Awadallah A, Sunoqrot S, Tarawneh O, Nazzal S, AlBaraghthi T, Sayyad JA, Abbas A. pH-dependent solubility and dissolution behavior of carvedilol—case example of a weakly basic BCS class II drug. AAPS PharmSciTech. 2016;17:418–26.
Singh B, Singh R, Bandyopadhyay S, Kapil R, Garg B. Optimized nanoemulsifying systems with enhanced bioavailability of carvedilol. Colloids Surf B Biointerface. 2013;101:465–74.
Wessler JD, Grip LT, Mendell J, Giugliano RP. The P-glycoprotein transport system and cardiovascular drugs. J Am Coll Cardiol. 2013;61:2495–502.
Venishetty VK, Chede R, Komuravelli R, Adepu L, Sistla R, Diwan PV. Design and evaluation of polymer coated carvedilol loaded solid lipid nanoparticles to improve the oral bioavailability: a novel strategy to avoid intraduodenal administration. Colloids Surf B Biointerface. 2012;95:1–9.
Kuentz M. Lipid-based formulations for oral delivery of lipophilic drugs. Drug Discov Today Technol. 2012;9:e97–104.
Rao S, Tan A, Thomas N, Prestidge CA. Perspective and potential of oral lipid-based delivery to optimize pharmacological therapies against cardiovascular diseases. J Control Release. 2014;193:174–87.
Chakraborty S, Shukla D, Mishra B, Singh S. Lipid—an emerging platform for oral delivery of drugs with poor bioavailability. Eur J Pharm Biopharm. 2009;73:1–15.
O’Driscoll CM. Lipid-based formulations for intestinal lymphatic delivery. Eur J Pharm Sci. 2002;15:405–15.
Kalepu S, Manthina M, Padavala V. Oral lipid-based drug delivery systems—an overview. Acta Pharm Sin B. 2013;6:361–72.
Pouton CW, Porter CJH. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev. 2008;60:625–37.
Dahan A, Hoffman A. The effect of different lipid based formulations on the oral absorption of lipophilic drugs: the ability of in vitro lipolysis and consecutive ex vivo intestinal permeability data to predict in vivo bioavailability in rats. Eur J Pharm Biopharm. 2007;67:96–105.
Mohsin K. Design of lipid-based formulations for oral administration of poorly water-soluble drug fenofibrate: effects of digestion. AAPS PharmSciTech. 2012;13:637–46.
Zhang J, Lv Y, Zhao S, Wang B, Tan M, Xie H, Lv G, Ma X. Effect of lipolysis on drug release from self-microemulsifying drug delivery systems (SMEDDS) with different core/shell drug location. AAPS PharmSciTech. 2014;15:731–40.
Bonnaire L, Sandra S, Helgason T, Decker EA, Weiss J, McClements DJ. Influence of lipid physical state on the in vitro digestibility of emulsified lipids. J Agric Food Chem. 2008;56:3791–7.
Witzleb R, Müllertz A, Kanikanti V-R, Hamann H-J, Kleinebudde P. Dissolution of solid lipid extrudates in biorelevant media. Int J Pharm. 2012;422:116–24.
Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Compr Rev Food Sci Food Saf. 2006;5:169–86.
Shahidi F, Zhong Y. Lipid oxidation and improving the oxidative stability. Chem Soc Rev. 2010;39:4067–79.
Strickley RG, Anderson BD. Solubilization and stabilization of an anti-HIV thiocarbamate, NSC 629243, for parenteral delivery, using extemporaneous emulsions. Pharm Res. 1993;10:1076–82.
Grove M, Mϋllertz A, Nielsen JL, Pedersen GP. Bioavailability of seocalcitol II: development and characterisation of self-microemulsifying drug delivery systems (SMEDDS) for oral administration containing medium and long chain triglycerides. Eur J Pharm Sci. 2006;28:233–42.
Ballard JM, Zhu L, Nelson ED, Seburg RA. Degradation of vitamin D3 in a stressed formulation: the identification of esters of vitamin D3 formed by a transesterification with triglycerides. J Pharm Biomed Anal. 2007;43:142–50.
Stella VJ. Chemical drug stability in lipids, modified lipids, and polyethylene oxide-containing formulations. Pharm Res. 2013;30:3018–28.
Chakraborty S, Shukla D, Vuddanda PR, Mishra B, Singh S. Effective in vivo utilization of lipid-based nanoparticles as drug carrier for carvedilol phosphate. J Pharm Pharmacol. 2011;63:774–9.
Shah MK, Madan P, Lin S. Preparation, in vitro evaluation and statistical optimization of carvedilol-loaded solid lipid nanoparticles for lymphatic absorption via oral administration. Pharm Dev Technol. 2014;19:475–85.
Wei L, Sun P, Nie S, Pan W. Preparation and evaluation of SEDDS and SMEDDS containing carvedilol. Drug Dev Ind Pharm. 2005;31:785.
Mahmoud EA, Bendas ER, Mohamed MI. Preparation and evaluation of self-nanoemulsifying tablets of carvedilol. AAPS PharmSciTech. 2009;10:183–92.
Singh B, Khurana L, Bandyopadhyay S, Kapil R, Katare OOP. Development of optimized self-nano-emulsifying drug delivery systems (SNEDDS) of carvedilol with enhanced bioavailability potential. Drug Deliv. 2011;18:599–612.
Borba PAA, Vecchia DD, Riekes MK, Pereira RN, Tagliari MP, Silva MAS, Cuffini SL, Campos CEM, Stulzer HK. Pharmaceutical approaches involving carvedilol characterization, compatibility with different excipients and kinetic studies. J Therm Anal Calorim. 2014;115:2507–15.
Silva LAD, Teixeira FV, Serpa RC, Esteves NL, Santos RR, Lima EM, Cunha-Filho MSS, Araújo AAS, Taveira SF, Marreto RN. Evaluation of carvedilol compatibility with lipid excipients for the development of lipid-based drug delivery systems. J Therm Anal Calorim. 2016;123:2337–44.
Tan CP, Che Man YB. Differential scanning calorimetric analysis of edible oils: comparison of thermal properties and chemical composition. J Am Oil Chem Soc. 2000;77:143–56.
Qi B, Zhang Q, Sui X, Wang Z, Li Y, Jiang L. Differential scanning calorimetry study—assessing the influence of composition of vegetable oils on oxidation. Food Chem. 2016;194:601–7.
ICH Guidance for Industry, Q2B, Validation of analytical procedures: methodology, international conference on harmonization. Geneva, 1996.
Tan CP, Che Man YB, Selamat J, Yusoff MSA. Application of arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. J Am Oil Chem Soc. 2001;78:1133–8.
Larsen DB, Parshad H, Fredholt K, Larsen C. Characteristics of drug substances in oily solutions. Drug release rate, partitioning and solubility. Int J Pharm. 2002;232:107–17.
Talvani A, Bahia MT, Sá-Barreto LCL, Lima EM, Cunha-Filho MSS. Carvedilol: decomposition kinetics and compatibility with pharmaceutical excipients. J Therm Anal Calorim. 2014;115:2501–6.
Stott PW, Williams AC, Barry BW. Mechanistic study into the enhanced transdermal permeation of a modelβ-blocker, propranolol, by fatty acids: a melting point depression effect. Int J Pharm. 2001;219:161–76.
Gallo RC, Ferreira APG, Castro REA, Cavalheiro ETG. Studying the thermal decomposition of carvedilol by coupled TG-FTIR. J Therm Anal Calorim. 2016;123:2307–12.
Hovorka SW, Schöneich C. Oxidative degradation of pharmaceuticals: theory, mechanisms and inhibition. J Pharm Sci. 2001;90:253–69.
Wasylaschuk WR, Harmon PA, Wagner G, Harman AB, Templeton AC, Xu H, Reed RA. Evaluation of hydroperoxides in common pharmaceutical excipients. J Pharm Sci. 2007;96:107–16.
Adhvaryu A, Erhan SZ, Liu ZS, Perez JM. Oxidation kinetic studies of oils derived from unmodifed and genetically modifed vegetables using pressurized differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim Acta. 2000;364:87–97.
Vecchio S, Cerretani L, Bendini A, Chiavaro E. Thermal Decomposition study of monovarietal extra virgin olive oil by simultaneous thermogravimetry/differential scanning calorimetry: relation with chemical composition. J Agric Food Chem. 2009;57:4793–800.
Halbaut L, Barbé C, Aróztegui M, Torre C. Oxidative stability of semisolid excipient mixtures with corn oil and its implication in the degradation of vitamin A. Int J Pharm. 1997;147:31–40.
Nishikawa M, Fujii K. Effect of autoxidation of hydrogenated castor oil containing 60 oxyethylene groups on degradation of miconazole. Chem Pharm Bull. 1991;39:2408–11.
Hartauer KJ, Arbuthnot GN, Baertschi SW, Johnson RA, Luke WD, Pearson NG, Rickard EC, Tingle CA, Tsang PKS, Wiens RE. Influence of peroxide impurities in povidone and crospovidone on the stability of raloxifene hydrochloride in tablets: identification and control of an oxidative degradation product. Pharm Dev Technol. 2000;5:303–10.
Waterman KC, Adami RC. Accelerated aging: prediction of chemical stability of pharmaceuticals. Int J Pharm. 2005;293:101–25.
Acknowledgements
The authors would like to thank the IQUEGO (Indústria Química do Estado de Goiás) for providing carvedilol.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, L.A.D., Cintra, E.R., Alonso, E.C.P. et al. Selection of excipients for the development of carvedilol loaded lipid-based drug delivery systems. J Therm Anal Calorim 130, 1593–1604 (2017). https://doi.org/10.1007/s10973-017-6380-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6380-7