Abstract
Nanofluids are a class of fluids prepared by dispersing nanoparticles in conventional base fluids. Owing to their excellent thermo-physical properties, nanofluids find potential applications in manufacturing industries. They are introduced to overcome the limitation with using traditional base fluids like water having low thermal conductivity (~ 0.612 W/mK at room temperature). The thermal conductivity of a base fluid is considerably increased by adding a modest number of nanoparticles to it. In the present work, we have prepared silver nanoparticles and nanorods using the simple chemical reduction method. UV–Visible spectroscopy and field emission scanning electron microscopy were used to investigate the optical characteristics and morphology of the produced nanomaterials. Furthermore, the effect of volume loadings of produced nanomaterials (0, 2%, 4%, 6%), as well as temperature on the thermal conductivity of the base fluids was investigated. The results are compared to different silver nanoparticles (AgNPs) loadings in the base fluid. Both silver nanoparticles and nanorods have optimal heat conductivity at 2 vol%. It is interesting to note that fluids with silver nanorods (AgNRs) portrayed better results compared to nanoparticles and the maximum enhancement observed of 78.4% for AgNRs-based nanofluids at temperature 323 K, which is very high when compared to most of the previously reported values.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Continuous progress in the production of robust engines in the manufacturing industries necessitates the use of highly efficient cooling systems. Cooling technology mainly aims to improve heat transfer, which requires the coolant to have excellent thermo-physical properties [1]. Conventional fluids, for instance water and ethylene glycol, used in heat exchangers have poor thermal conductivity (TC) [2]. To combat this challenge nanofluids have been employed, which contain solid nanoparticles of size ≤ 100 nm that are dispersed into a base fluid [3]. As nanofluids have greater number of particles per unit volume, they portray better TC than conventional fluids. Several factors, such as nature of the material, temperature, volume fraction of nanoparticles dispersed, viscosity, aspect ratio, type of base fluid, and the stabilizer [4] affect the nanofluid’s thermal conductivity. Studies have confirmed that in most of the cases, with an increase in particle concentration, and temperature, TC has increased [5]. Binary conventional base fluids, for example a combination of water and ethylene glycol, resulted in slight enhancement in heat transfer properties when compared to monofluids. However, these cannot be used in systems, which produce high heat flux and it can be resolved by using oil-based nanofluids [6].
It is interesting to note that trivial addition of nanoparticles dramatically improved the TC of a base fluid. When nanofluids are used as a coolant in heat exchanger, nanomaterials enhance the heat absorption. Due to varying density, heat is uniformly dissipated across the fluid. This implies that when the number of particles is higher, the direct contact of particles with the hot surface results in enhanced TC. But the experimental results have also indicated that TC is higher at a particular volume concentration and with further rise in concentration leads to cause a decrement in TC. Stephen U.S. Choi and J. A. Eastman were the first to suggest dispersing nanometer-sized metallic particles in traditional heat fluids to generate a new class of engineering fluids with improved thermal conductivity, dubbed nanofluids. They theoretically examined the influence of copper nanoparticles suspended in water on thermal conductivity of nanofluids. The use of nanofluids reduced heat exchanger pumping power by a significant amount [7]. Later, nanofluids based on various nanomaterials such as Cu NPs [8], CuO nanowires [9], Ag NPs [10], graphene [11], multiwalled CNTs [12], and MXenes [13] were successfully synthesized and showed improved thermal conductivity when compared to the corresponding base fluid, bringing hope of increasing engine efficiency.
Sivakumar et al. created a forced convection solar dryer integrated with a CuO nanoparticle-coated flat plate solar collector to investigate the effectiveness of drying maize under Coimbatore's meteorological conditions. The modified dryer's performance was tested at a constant air flow rate of 1.5 m3/min, and the results were compared to the conventional type. In the presence of nanoparticles, the collector's efficiency increased by 4% [14]. The thermal performance of a heat pipe that employs nano-enhanced phase change material (PCM) as an energy storage medium for electronic cooling applications was investigated. The thermal conductivity of the nano-enhanced PCM is measured and found to be increased by up to 32% when compared to pure Tricosane. The nano-enhanced PCM can store nearly 30% of the energy supplied at the evaporator, resulting in a reduction in fan power consumption [15]. Heat transfer performance of a computer cooling system using a water cooling kit with nanofluids was studied. The effect of nanofluids on the cooling system in real-world conditions was determined using a real computer setup with a quad core processor. Thermal conductivity and convection coefficients are improved over pure fluid values, the flow rate required to meet the economy and power consumption of the cooling system [16].
In our previous work, polyvinyl alcohol (PVA), polyvinyl pyrrolidone (PVP), and blend (PVA/PVP) polymer dispersant-stabilized Ag nanofluids are prepared. At an equimolar ratio of blend polymer dispersant for a lower Ag particle concentration (0.005 percent), a 31.49 percent increase in thermal conductivity was found [17]. Similarly, the AuAg and CuAg bimetallic NPs, prepared via seed colloidal technique showed improved thermal conductivity (8% and 7%) when compared to base fluid (water) [18].
Observations suggest that the shape-dependent studies on thermal conductivity of nanomaterials are sparse. As a result, a study of the influence of morphology on heat conductivity is required. In this vein, the current research aims to investigate the feasibility of modifying the TC for heat transfer applications by changing the structure of the nanomaterial.
Specifics of the experiment
Chemicals used
Silver nitrate (AgNO3, 99%), cetyltrimethylammonium bromide (CTAB, 99%), and sodium hydroxide (NaOH, 99%) are obtained from Loba Chemie Pvt Ltd. Ascorbic acid (C6H8O6, 99%) and sodium borohydride (NaBH4, 98%) are bought from Sigma-Aldrich. All the chemicals are used without being purified further.
Synthesis
Silver nanorods of different aspect ratio were synthesized using seed-mediated procedure [19].
Preparation of seed solution 10 µl AgNO3 (0.01 M) and 80 µl CTAB (0.1 M) were mixed, and the resultant solution was diluted and made up to 20 mL using distilled water and 0.6 mL NaBH4 (0.01 M) was added. CTAB and NaBH4 serve as capping and reducing agents, respectively, in this reaction. After that, the solution was swirled for 2 min. Before addition to the growth solution, the prepared seed solution was kept undisturbed for roughly an hour.
Preparation of growth solution A 10 mL growth solution was made up of 9.25 mL of CTAB (0.01 M), 0.25 mL of AgNO3 (0.02 M), and 0.5 mL of ascorbic acid (0.1 M). 0.125 mL seed solution was added to the resulting mixture, followed by 0.1 mL NaOH (1 M) to prepare Ag NRs. Formation of pinkish purple colloidal solution represents the formation of nanorods.
Nanofluids preparation For the preparation of nanofluids of required concentration, the colloidal solution of NPs and NRs was dispersed in distilled water to obtain different volume loadings of nanorods (0%, 2%, 4%, 6%).
Characterization
The structure of the silver nanorods is analyzed using a field emission scanning electron microscope (FESEM; Carl Zeiss; EVO-18). The SHIMADZU-1800 UV–Visible spectrophotometer was operated to study the optical properties of silver nanoparticles and nanorods. Decagon Devices, Inc.'s KD2 pro setup was used to evaluate thermal conductivity.
Outcomes and discussion
Optical and morphological properties
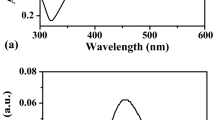
Among the most important optical properties of metallic nanoparticles is surface plasmon resonance (SPR). It is well known that the nature of the material, size, shape, composition, and local environment of the synthesized nanoparticles dictates the optical spectra. The absorption spectra of Ag NPs (seed solution) and Ag NRs are shown in Fig. 1. The silver seed solution displayed a single absorbance peak at 403 nm, suggesting the development of spherical nanoparticles. Two distinctive peaks at 545 nm and 410 nm, associated with the longitudinal surface plasmon peak and the transverse surface plasmon peak, respectively, shows the successful formation of silver nanorods.
The FESEM studies showed the presence of both Ag nanoparticles and nanorods (in Fig. 2b) in final product, whereas the seed solution contained only silver nanoparticles of average size ~ 13 nm as depicted in Fig. 2a. Silver nanorods of varying dimensions are observed in Fig. 2b, having an average length and diameter of ~ 29 nm and ~ 16 nm respectively, and the average aspect ratio was determined to be 1.79 correspondingly.
Thermal conductivity studies
The transient hot wire (THW) method is a widely used method to determine the thermal conductivity of liquids, gases, and solids, and it may also be used to investigate the TC of nanofluids. KD2 Pro setup operates based on this technique, where TC can be determined by observing the change in temperature over time at a fixed distance from a heating wire immersed in test sample. Using the following expression, KD2 Pro gives the thermal conductivity [20]. The technique is based on recording the transient temperature rise of a thin vertical metal wire with infinite length when a step voltage is applied to it. The wire is immersed in a fluid and can act both as an electrical heating element and a resistance thermometer. The temperature gradient between the wire surface and the fluid generates a buoyant force, resulting in natural convection, which tends to measure fluid thermal conductivity. The thermal conductivity was calculated from the slope of the rise in the wire's temperature against the logarithmic time interval. The uncertainty of this measurement is estimated to be within ± 1.0% [21].
where q denotes the constant heat rate and the changes in temperature with time t1 and t2 are denoted using \(\Delta T_{1}\) and \(\Delta T_{2}\).
The following equation [10] can be used to compute the percentage of thermal conductivity enhancement, i.e.,
Here knf and kbf are used to denote the thermal conductivities of nanofluid as well as the base fluid at a particular temperature.
Thermal conductivity tests were performed on a variety of volume concentrations (0%, 2%, 4%, 6%) of Ag NRs and Ag NPs (Fig. 3). Upon the introduction of a small volume concentration of Ag NPs and NRs (2 vol%), significant enhancement in TC, i.e., 49.42% and 65.96%, respectively, was observed at room temperature. Different methods can be used to explain the enhancement in TC, including Brownian motion of the particles, ballistic heat transport, molecular stacking at the nanoparticle–liquid interface, and NP cluster formation. However, further increase in concentration results in reduction of TC and approaches to TC of the base fluid. The above effect has been attributed to the agglomeration caused by the formation of closely packed clusters [22].
The effect of temperature variation on thermal conductivity was also studied in the range of 306–323 K, shown in Fig. 4. The thermal conductivity of the nanofluid increased as the temperature went up. When thermal conductivity of nanofluids comprising only NPs and nanofluids containing NRs was compared, fluids containing nanorods exhibited a superior thermal conductivity regardless of temperature. Ag NRs-based nanofluid exhibited maximum thermal conductivity enhancement (78.4%) with respect to base fluid at 323 K, which is much superior compared to most of the reported values (Table 1).
Moreover, spherical Ag NPs-based nanofluid also showed remarkable enhancement at all the temperature with respect to the base fluid, with a maximum increment (53.3%) at 313 K, which is notable. This might be due to the formation of very small sized (≤ 20 nm) nanoparticles [23]. Furthermore, diffusive heat conduction dominates in NRs-based nanofluids, whereas thermal conductivity improvement in NPs-based nanofluids is primarily due to Brownian motion of the particles. The dominance of diffusive heat conduction mechanism improves with the increasing aspect ratio [24]. The diffusive heat conduction depends upon the interfacial thermal resistance Ri. The interfacial thermal resistance hinders heat diffusion due to solid–liquid interactions. With the enhancement in concentration and temperature, the Ri values decrease, allowing more heat transfer through thermal diffusion. As a result, diffusive heat conduction in nanofluids increases. The relaxation time is significantly affected by the long-time tail in Brownian motion of the nanoparticles occurred because of the inertia of the moving nanoparticles. Thus, the particles need more time to adjust their movement between two successive collisions. Therefore, the individual contribution due to Brownian motion gets reduced. Moreover, the ratio of heat transfer by the moving nanoparticles due to Brownian motion to the same with base fluid is negligible. However, with increase in temperature, agitation among nanoparticles increases which enhances Brownian motion of nanoparticles [25].
The increased surface area-to-volume ratio of nanorods, which results in a larger contact area of the NPs with the base fluid, can be linked to their improved heat conductivity when compared to nanoparticles [26].
Table 1 shows the TC of nanofluids containing nanomaterials with different morphologies. It is evident that when compared to spherical nanoparticles, one-dimensional and two-dimensional nanomaterial-based nanofluids possess interesting thermal properties, which is highly advantageous in heat exchangers. Further, due to the presence of covalent intra-layer bonds as well as Van der Waals layer-to-layer interactions, 2D-layered material-based nanofluid exhibits significant increase in TC.
Comparison of experimental results with the classical theoretical models
Xuan et al. [27] and Kumar et al. [28] provided classical models that were compared to the experimentally obtained thermal conductivity of nanofluids. These models describe the temperature and Brownian motion of the NPs as factors of thermal conductivity of nanofluids.
Xuan et al. developed a modified formula from the Maxwell model for effective thermal conductivity of nanofluids [29], i.e.,
where \(k_{{\text{B}}}\) = Boltzmann constant (1.381 × 10−23 J K−1), \(r_{{\text{c}}}\)—apparent radius of cluster (35 Å), \(k_{{\text{p}}}\) (191.32 W m−1 K−1) and (0.599 W m−1 K−1), \(k_{{\text{p}}}\) and \(k_{{\text{b}}}\) are the thermal conductivity of the NPs and base fluid, respectively, \(C_{{\text{p}}}\)—specific heat capacity (4.186 J kg−1 °C−1), respectively.
Kumar et al. proposed a comprehensive model to evaluate the effective thermal conductivity of nanofluids by combining two sub-classical models, i.e., stationary particle model derived from the Fourier’s law of diffusion and the moving particle deduced from the Stokes–Einstein formula following the strong temperature dependence for the enhancement of thermal conductivity in nanofluids. It is expressed as [30],
where \(C_{{\text{p}}}\) is heat constant (4.186 J kg−1 °C−1), \(\vartheta\)—dynamic viscosity of the base fluid (9.375 × 10−4 Pa·s), and dp—diameter of the particles (Fig. 5).
In comparison with the Kumar et al. and Xuan et al. models, at lower temperatures there was clear agreement of experimental findings with the Xual et al. model for both Ag NPs and Ag NRs; however, there was some departure at higher temperatures.
Conclusions
The seed-mediated growth method was used to successfully produce silver nanorods. The formation of nanorods is evident from the second peak observed at 545 nm, whereas for spherical nanoparticles the peak was observed at 410 nm in optical spectra. From the morphological studies it was observed that there was non-uniform distribution of nanorods having average aspect ratio 1.79 along with the formation of spherical nanoparticles. The TC experiments revealed that when the vol% of silver NPs/NRs increased, the TC dropped due to nanoparticle agglomeration at greater concentrations. The influence of temperature on nanofluid TC was also investigated, and it was discovered that as temperature rose, so did TC. Both Ag NR and NP-based nanofluids showed remarkable enhancement (53.3% and 78.4%) at temperatures 313 and 323 K. Hence, further studies on rheological properties and controlled morphology of Ag NR based nanofluids can make them to be used as heat transfer fluids in industries.
References
Zhang L, Yu W, Zhu D, Xie H, Huang G. Enhanced thermal conductivity for nanofluids containing silver nanowires with different shapes. J Nanomater. 2017;2:1–6.
Sivashanmugam P. Application of nanofluids in heat transfer. An overview. Heat Transf Phenom 2012;16.
Wen D, Ding Y. Experimental investigation into convective heat transfer of nanofluids at the entrance region under laminar flow conditions. Int J Heat Mass Transf. 2004;47:5181–8.
Halelfadl S, Maré T, Estellé P. Efficiency of carbon nanotubes water based nanofluids as coolants. Exp Therm Fluid Sci. 2014;53:104–10.
Iyahraja S, Rajadurai JS. Study of thermal conductivity enhancement of aqueous suspensions containing silver nanoparticles. AIP Adv. 2015;5:3–11.
Sarafraz MM, Arya A, Nikkhah V, Hormozi F. Thermal performance and viscosity of biologically produced silver/coconut oil nanofluids. Chem Biochem Eng Q. 2016;30:489–500.
Choi SUS, Eastman JA. Enhancing thermal conductivity of fluids with nanoparticles. Am Soc Mech Eng Fluids Eng Div FED. 1995;231:99–105.
Yu W, Xie H, Chen L, Li Y. Investigation on the thermal transport properties of ethylene glycol-based nano fluids containing copper nanoparticles. Powder Technol. 2010;197:218–21.
Zhu D, Wang L, Yu W, Xie H. Intriguingly high thermal conductivity increment for CuO nanowires contained nanofluids with low viscosity. Sci Rep. 2018;8:1–12.
Khamliche T, Khamlich S, Doyle TB, Makinde D, Maaza M. Thermal conductivity enhancement of nano-silver particles dispersed ethylene glycol based nanofluids. Mater Res Express. 2018;5:035020.
Yu W, Xie H, Wang X, Wang X. Significant thermal conductivity enhancement for nanofluids containing graphene nanosheets. Phys Lett A. 2011;375:1323–8.
Ding Y, Alias H, Wen D, Williams RA. Heat transfer of aqueous suspensions of carbon nanotubes (CNT nanofluids). Int J Heat Mass Transf. 2006;49:240–50.
Bao Z, Bing N, Zhu X, Xie H, Wei Yu. Ti3C2Tx, MXene contained nanofluids with high thermal conductivity, super colloidal stability and low viscosity. Chem Eng J. 2021;406:126390.
Sivakumar S, Velmurugan C, Jacob DSE, Solomon AB, Dev KL. Effect of nano cupric oxide coating on the forced convection performance of a mixed-mode flat plate solar, dryer. Renew Energy. 2020;155:1165–72.
Krishna J, Kishore PS, Solomon AB. Heat pipe with nano enhanced-PCM for electronic cooling application. Exp Therm Fluid Sci. 2017;81:84–92.
Rafati M, Hamidi AA, Niaser MS. Application of nano fluids in computer cooling systems. Appl Ther Eng. 2012;46:9–14.
Pavithra KS, et al. Polymer-dispersant-stabilized Ag nanofluids for heat transfer applications. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-10064-8.
Dsouza A, et al. CuAg and AuAg bimetallic nanoparticles for catalytic and heat transfer applications. Clean Technol Environ Policy. 2021. https://doi.org/10.1007/s10098-021-02120-0.
Rekha CR, Nayar VU, Gopchandran KG. Synthesis of highly stable silver nanorods and their application as SERS substrates. J Sci: Adv Mater Devices. 2018;3:196–205.
Kleinstreuer C, Feng Y. Experimental and theoretical studies of nanofluid thermal conductivity enhancement: a review. Nanoscale Res Lett. 2011;6:229.
Xie H, Wei Yu, Li Y, Chen L. Discussion on the thermal conductivity enhancement of nanofluids. Nanoscale Res Lett. 2011;6:124.
Ceylan A, Jastrzembski K, Shah SI. Enhanced solubility Ag-Cu nanoparticles and their thermal transport properties. Metall Mater Trans A: Phys Metall Mater Sci. 2006;37:2033–8.
Masuda H, Ebata A, Teramae K. Alteration of thermal conductivity and viscosity of liquid by dispersing ultra-fine particles. Dispersion of Al2O3, SiO2 and TiO2 ultra-fine particles. J Jpn Soc Thermophys Prop. 1993;7:227–33.
Yang B, Han Z. Temperature-dependent thermal conductivity of nanorod-based nanofluids. Appl Phys Lett. 2006;89:083111.
Chakraborty S, et al. Contributory effect of diffusive heat conduction and Brownian motion on thermal conductivity enhancement of nanofluids. J Phys. 2020;94:150.
Qiu L, et al. A review of recent advances in thermophysical properties at the nanoscale: from solid state to colloids. Phys Rep. 2020;843:1–81.
Rostami S, Kalbasi R, Talebkeikhah M, Goldanlou AS. Improving the thermal conductivity of ethylene glycol by addition of hybrid nano-materials containing multi-walled carbon nanotubes and titanium dioxide: applicable for cooling and heating. J Therm Anal Calorim. 2021;143:1701–12.
Michael M, Zagabathuni A, Sikdar S, Pabi SK, Ghosh S. Effect of dispersion behavior on the heat transfer characteristics of alumina nanofluid: an experimental investigation and development of a new correlation function. Int Nano Lett. 2020;10:207–17.
Islam MR, Shabani B, Rosengarten G. Nanofluids to improve the performance of PEM fuel cell cooling systems: a theoretical approach. Appl Energy. 2016;178:660–71.
Hussein AM, Sharma KV, Bakar RA, Kadirgama K. The effect nanofluid volume concentration on heat transfer and friction factor inside a horizontal tube. J Nanomater. 2013;2013:1.
Acknowledgements
Smita Mahadevappa Nyamgoudar, Vasavi Prasuna Silaparasetti Shilpa M P, Gurumurthy S C, and Ganesha A acknowledge Manipal Academy of Higher Education for the funding facility. Authors are grateful to Mangalore University DST-PURSE laboratory for providing the FESEM facility. The authors Koduri Ramam and Srivathsava Surabhi are greatly indebted to Universidad de Concepción (UdeC), Chile, for its huge support and facilities. We also acknowledge the corresponding Fondecyt Regular (No 1140420) and postdoctoral (No 3200832) Projects Programa Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) de la Agencia Nacional de Investigación y Desarrollo, Chile. The authors acknowledge Vishwanath H S for designing graphical abstract.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Contributions
The study was designed and planned by SMN, KR and SCG. SMN, VPS, and MPS carried out the experiment, analyzed the data and wrote the Manuscript. KSP and SS carried out theoretical simulations. AG helped in carrying out experiments as well as revising the manuscript. SM,R, and KME helped in data curation and Analysis. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nyamgoudar, S.M., Silaparasetti, V.P., Shilpa, M.P. et al. Analysis of shape dependency of thermal conductivity of silver-based nanofluids. J Therm Anal Calorim 147, 14031–14038 (2022). https://doi.org/10.1007/s10973-022-11604-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11604-0