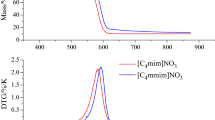
Abstract
Ionic liquids (ILs) can be used as building materials, such as ILs polymers. Thermal stability is the basic attribute of selecting the most suitable high-temperature lubricant, fluid, and solvent compound for high-temperature organic reactions. The related literatures showing the thermal hazard of ILs at high temperature have been explored, but the analysis basis and evaluation reaction mode are lacking. This study combines reliable literature values of reaction parameters that no need for considering the complexity of reaction mode and nonlinear fitting that can calculate advanced reaction mode to follow the past literature flow and establish subsequent hazardous properties on ILs. A frequently used ILs, 1-butyl-3-methylimidazolium nitrate ([Bmmim]NO3), was selected and measured by thermogravimetric analyzer. The weight loss data recorded by the instrument are combined with the thermodynamic equation to ascertain the reaction kinetics of [Bmmim]NO3. The kinetics is described the changes and trends of the overall reaction on which the influence of external temperature is brought into reaction system. The numerical model is constructed to evaluate the thermal hazards of a large number of substances in the actual environment with different container forms in 25.0 g and 50.0 g packages. The results show that [Bmmim]NO3 has briefer period (< 1 h) for maximum reaction rate when the temperature is higher than 300 °C. The safety temperature of the reaction rate change is higher, and the temperature change of the runaway reaction is similar to that of the previous literature, respectively. Even the emergency response temperature range of the reaction rate is wider, attention should still be paid to the hazard of the runaway reaction at high temperature (> 270 °C).






Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor of the Arrhenius equation/s–1
- C p :
-
Specific heat capacity/J (g K)–1
- DTG :
-
Mass loss rate which determined by TGA/% min–1
- E a :
-
Apparent activation energy/kJ mol–1
- f(α):
-
Kinetics function/dimensionless
- i :
-
Component number/dimensionless
- n :
-
Reaction order/dimensionless
- R :
-
Gas constant/J (mol K)–1
- r :
-
Reaction rate constant/mol (L s)–1
- t :
-
Time/s or min
- T :
-
Temperature of sample/°C
- TG:
-
Mass loss which determined by TGA/%
- TCL:
-
Time to conversion limit/day
- T 0 :
-
Apparent exothermic onset temperature/°C
- TMR:
-
Time to maximum rate/min
- TMRad :
-
Time to maximum rate under adiabatic conditions/min
- TMRiso :
-
Time to maximum rate under isothermal conditions/min
- U :
-
Heat transfer coefficient/W m–2 K–1
- W :
-
Heat generation/J s–1
- X :
-
The normal to the surface of an object/dimensionless
- α :
-
Conversion degree of a component/dimensionless
- β :
-
Heating rate/°C min–1
- λ :
-
Heat conductivity/W (m K)–1
- ρ :
-
Density/kg m–3
References
Liu SH, Zhang B. Using thermal analysis technology to assess the thermal stability of 1,3-dimethylimidazolium nitrate. Process Saf Environ Prot. 2019;124:181–6. https://doi.org/10.1016/j.psep.2019.02.012.
Matandabuzo M, Ajibade PA. Vinyl pyridinium polymeric ionic liquid functionalized carbon nanotube composites as adsorbent for chromium(VI) in aqueous solution. J Mol Liq. 2019;296: 111778. https://doi.org/10.1016/j.molliq.2019.111778.
Nair JR, Colò F, Kazzazi A, Moreno M, Bresser D, Lin R, et al. Room temperature ionic liquid (RTIL)-based electrolyte cocktails for safe, high working potential Li-based polymer batteries. J Power Sources. 2019;412:398–407. https://doi.org/10.1016/j.jpowsour.2018.11.061.
Zhu G, Cheng G, Lu T, Cao Z, Wang L, Li Q, et al. An ionic liquid functionalized polymer for simultaneous removal of four phenolic pollutants in real environmental samples. J Hazard Mater. 2019;373:347–58. https://doi.org/10.1016/j.jhazmat.2019.03.101.
Abushammala H, Mao J. A review on the partial and complete dissolution and fractionation of wood and lignocelluloses using imidazolium ionic liquids. Polymers. 2020;12(1):195.
Vekariya RL. A review of ionic liquids: applications towards catalytic organic transformations. J Mol Liq. 2017;227:44–60. https://doi.org/10.1016/j.molliq.2016.11.123.
Singh SK, Savoy AW. Ionic liquids synthesis and applications: an overview. J Mol Liq. 2020;297: 112038. https://doi.org/10.1016/j.molliq.2019.112038.
Hullmann A. The economic development of nanotechnology-an indicators based analysis. EU report. 2006.
Consultancy HK. Ionic liquids 2030—ionic liquid technologies, markets, applications, companies and developments worldwide 2008 to 2020 and 2030. Tübingen, Germany. Tübingen. 2012.
Markets and Markets Research. Ionic liquids market by application (solvents & catalysts, process & operating fluids, plastics, batteries & electrochemistry, bio-refineries) and by region (North America, Europe, Asia-Pacific and rest of World) - global forecast to 20212016.
Frietsch R, Kladroba A, Markianidou P, Neuhäusler P, Peter V, Ravet J et al. Final Report on the Collection of Patents and Business Indicators by Economic Sector: Societal Grand Challenges and Key Enabling Technologies Collection and Analysis of Private R&D Investment and Patent Data in Different Sectors, Thematic Areas and Societal Challenges. Publications Office of the European Union; 2017.
Schubert TJS. Current and future ionic liquid markets. In: Shiflett MB, Scurto AM, editors. Ionic liquids: current state and future directions. Washington, DC: American Chemical Society; 2017. p. 35–65. https://doi.org/10.1021/bk-2017-1250.ch003.
Singh NR, Speight JG. Applications of ionic liquids in industry. Chem Technol Indian J. 2011;6(2):114–22.
Han S, Li J, Zhu S, Chen R, Wu Y, Zhang X, et al. Potential applications of ionic liquids in wood related industries. BioResources. 2009;4(2):825–34.
Bailey MP. Ionic liquids create more sustainable processes. Chem Eng. 2015;122(10):18.
Meksi N, Moussa A. A review of progress in the ecological application of ionic liquids in textile processes. J Clean Prod. 2017;161:105–26.
Ratti R. Ionic liquids: synthesis and applications in catalysis. Adv Chem. 2014;2014: 729842. https://doi.org/10.1155/2014/729842.
Werner S, Haumann M. Ultralow temperature water–gas shift reaction enabled by supported ionic liquid phase catalysts. Support Ion Liq Fundam Appl. 2014:327–50.
Plechkova NV, Seddon KR. Applications of ionic liquids in the chemical industry. Chem Soc Rev. 2008;37(1):123–50.
Kalb RS. Toward Industrialization of Ionic Liquids. In: Shiflett MB, editor. Commercial applications of ionic liquids. Cham: Springer International Publishing; 2020. p. 261–82. https://doi.org/10.1007/978-3-030-35245-5_11.
Kossoy AA, Akhmetshin YG. Simulation-based approach to design of inherently safer processes. Process Saf Environ Prot. 2012;90(5):349–56. https://doi.org/10.1016/j.psep.2012.03.007.
Duh YS, Kao CS, Lee WLW. Chemical kinetics on thermal decompositions of dicumyl peroxide studied by calorimetry. J Therm Anal Calorim. 2017;127(1):1089–98. https://doi.org/10.1007/s10973-016-5797-8.
Jayakumar M, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR. Electrochemical behavior of ruthenium (III), rhodium (III) and palladium (II) in 1-butyl-3-methylimidazolium chloride ionic liquid. Electrochim Acta. 2009;54(26):6747–55. https://doi.org/10.1016/j.electacta.2009.06.043.
Maton C, De Vos N, Stevens CV. Ionic liquid thermal stabilities: decomposition mechanisms and analysis tools. Chem Soc Rev. 2013;42(13):5963–77. https://doi.org/10.1039/C3CS60071H.
Liu SH, Zhang B, Cao CR. Evaluation of thermal properties and process hazard of three ionic liquids through thermodynamic calculations and equilibrium methods. J Loss Prev Process Ind. 2020;68: 104332. https://doi.org/10.1016/j.jlp.2020.104332.
Meng J, Pan Y, Ran Z, Li Y, Jiang J, Wang Q, et al. Thermal hazard and decomposition kinetics of 1-butyl-2,3-dimethylimidazolium nitrate via TGA/DSC and FTIR. J Loss Prev Process Ind. 2021;72: 104562. https://doi.org/10.1016/j.jlp.2021.104562.
Bonilla J, Salazar RP, Mayorga M. Kinetic triplet of Colombian sawmill wastes using thermogravimetric analysis. Heliyon. 2019;5(10):e02723. https://doi.org/10.1016/j.heliyon.2019.e02723.
Vimalathithan PK, Barile C, Vijayakumar CT. Investigation of kinetic triplets for thermal degradation of thermally cured vinyl ester resin systems and lifetime predictions. J Therm Anal Calorim. 2018;133(2):881–91. https://doi.org/10.1007/s10973-018-7154-6.
Kossoy AA, Akhmetshin YG. Identification of kinetic models for the assessment of reaction hazards. Process Saf Prog. 2007;26(3):209–20. https://doi.org/10.1002/prs.10189.
Liu SH, Shu CM. Advanced technology of thermal decomposition for AMBN and ABVN by DSC and VSP2. J Therm Anal Calorim. 2015;121(1):533–40. https://doi.org/10.1007/s10973-015-4559-3.
Liu SH, Hou HY, Shu CM. Thermal hazard evaluation of the autocatalytic reaction of benzoyl peroxide using DSC and TAM III. Thermochim Acta. 2015;605:68–76. https://doi.org/10.1016/j.tca.2015.02.008.
Martín-Lara M, Blázquez G, Zamora M, Calero M. Kinetic modeling of torrefaction of olive tree pruning. Appl Therm Eng. 2017;113:1410–8.
Li XR, Koseki H, Iwata Y, Mok YS. Decomposition of methyl ethyl ketone peroxide and mixtures with sulfuric acid. J Loss Prev Process Ind. 2004;17(1):23–8.
Zhu H, Liu N. Kinetic analysis based on the kinetic compensation effect and optimization calculation. Thermochim Acta. 2020;690: 178686. https://doi.org/10.1016/j.tca.2020.178686.
Chen WC, Shu CM. Prediction of thermal hazard for TBPTMH mixed with BPO through DSC and isoconversional kinetics analysis. J Therm Anal Calorim. 2016;126(3):1937–45.
Kossoy AA, Sheinman IY. Evaluating thermal explosion hazard by using kinetics-based simulation approach. Process Saf Environ Prot. 2004;82(6):421–30. https://doi.org/10.1205/psep.82.6.421.53208.
Kossoy AA, Benin AI, Akhmetshin YG. An advanced approach to reactivity rating. J Hazard Mater. 2005;118(1):9–17. https://doi.org/10.1016/j.jhazmat.2004.08.015.
Andreozzi R, Caprio V, Somma ID, Sanchirico R. Kinetic and safety assessment for salicylic acid nitration by nitric acid/acetic acid system. J Hazard Mater. 2006;134(1):1–7. https://doi.org/10.1016/j.jhazmat.2005.10.037.
Gonzales NO, Levin ME, Zimmerman LW. The reactivity of sodium borohydride with various species as characterized by adiabatic calorimetry. J Hazard Mater. 2007;142(3):639–46. https://doi.org/10.1016/j.jhazmat.2006.08.058.
Roduit B, Folly P, Sarbach A, Berger B, Brogli F, Mascarello F, et al. Estimation of time to maximum rate under adiabatic conditions (TMRad) using kinetic parameters derived from DSC-investigation of thermal behavior of 3-methyl-4-nitrophenol. Chem Propel Polym Mater. 2011;1:84–93.
United Nations. Recommendations on the transport of dangerous goods: model regulations (twentieth revised edition). New York, USA: United Nations Publications; 2018.
United Nations. Recommendations on the transport of dangerous goods: manual of tests and criteria-sixth. Revised. New York, USA: United Nations Publications; 2018.
Kossoy AA, Sheinman IY. Comparative analysis of the methods for SADT determination. J Hazard Mater. 2007;142(3):626–38.
Li XR, Koseki H. SADT prediction of autocatalytic material using isothermal calorimetry analysis. Thermochim Acta. 2005;431(1):113–6. https://doi.org/10.1016/j.tca.2005.01.051.
Malow M, Wehrstedt KD. Prediction of the self-accelerating decomposition temperature (SADT) for liquid organic peroxides from differential scanning calorimetry (DSC) measurements. J Hazard Mater. 2005;120(1):21–4. https://doi.org/10.1016/j.jhazmat.2004.12.040.
Steensma M, Schuurman P, Malow M, Krause U, Wehrstedt KD. Evaluation of the validity of the UN SADT H.4 test for solid organic peroxides and self-reactive substances. J Hazard Mater. 2005;117(2):89–102. https://doi.org/10.1016/j.jhazmat.2004.09.028.
Lv J, Chen L, Chen W, Gao H, Peng M. Kinetic analysis and self-accelerating decomposition temperature (SADT) of dicumyl peroxide. Thermochim Acta. 2013;571:60–3. https://doi.org/10.1016/j.tca.2013.08.029.
Kossoy AA, Belokhvostov VM, Koludarova EY. Thermal decomposition of AIBN: part D: verification of simulation method for SADT determination based on AIBN benchmark. Thermochim Acta. 2015;621:36–43. https://doi.org/10.1016/j.tca.2015.06.008.
Acknowledgements
The authors are grateful to the technical support from National Yunlin University of Science and Technology.
Author information
Authors and Affiliations
Contributions
C-FT rendered suggestions to modify the design of the experiments and wrote this manuscript and analyzed the experiments data. I-JW provided idea for using kinetic models to obtain the critical thermal safety parameters. All authors supplied comments on this theme.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsai, CF., Wen, IJ. Reaction mode on the green construction process and corresponding thermal stability evaluation of ionic liquid. J Therm Anal Calorim 147, 10745–10754 (2022). https://doi.org/10.1007/s10973-022-11314-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11314-7