Abstract
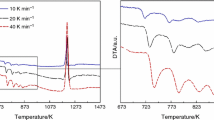
Metal ions, as common impurities in the crystallization process of explosives, have a significant influence on the crystal quality and performance. The effects of Fe3+, Zn2+, Ca2+ and Na+ ions on the crystallization and thermal properties of octogen (HMX) were studied. And the defect distribution as well as formation process of the explosive crystals doped with the metal ions were analysed. ICP, XPS and an etching method were utilized to characterize the contents and distributions of residual metal ions in the HMX crystals. The effects of the metal ions on the thermal decomposition and phase transition of HMX were obtained based on DSC-TG and in situ XRD. The mechanism of the influence of the metal ions on the crystallization of HMX was also explored. The results showed that the metal ions would change the nucleation growth mode of HMX to form dispersed crystalline HMX. The residual metal ions were mainly adsorbed on the crystal surface and included in the crystal. The metal ions had a significant influence on the thermal properties of HMX, with Ca2+ and Fe3+ inhibiting the thermally induced phase transition of HMX, Na+ promoting the phase transition of HMX, and the effect of Zn2+ on the phase transition of HMX depending on its concentration. The growth mechanism of doped HMX was lamellar stacking, and the metal ions changed the quality of the crystal by hindering the growth step of the HMX crystal. Since the crystal quality is highly related to the thermal performance, the safety of the explosive is affected.










Similar content being viewed by others
References
Hikal WM, Bhattacharia SK, Peterson GR, Weeks BL. Controlling the coarsening stability of pentaerythritol tetranitrate (PETN) single crystals by the use of water. Thermochim Acta. 2012;536:63–7.
Sinapour H, Damiri S, Pouretedal HR. The study of RDX impurity and wax effects on the thermal decomposition kinetics of HMX explosive using DSC/TG and accelerated aging methods. J Therm Anal Calorim. 2017;129(1):271–9.
Xu J, Zheng S, Huang S, Tian Y, Liu Y, Zhang H, Sun J. Host-guest energetic materials constructed by incorporating oxidizing gas molecules into an organic lattice cavity toward achieving highly-energetic and low-sensitivity performance. Chem Commun. 2019;55(7):909–12.
Yan QL, Zeman S, Elbeih A, Song ZW, Málek J. The effect of crystal structure on the thermal reactivity of CL-20 and its C4 bonded explosives (I): thermodynamic properties and decomposition kinetics. J Therm Anal Calorim. 2013;112(2):823–36.
Ravindran TR, Rajan R, Venkatesan V. Review of phase transformations in energetic materials as a function of pressure and temperature. J Phys Chem C. 2019;123(48):29067–85.
Xu J, Tian Y, Liu Y, Zhang H, Shu Y, Sun J. Polymorphism in hexanitrohexaazaisowurtzitane crystallized from solution. J Cryst Growth. 2012;354:13–9.
Kim Y, Ambekar A, Yoh JJ. Toward understanding the aging effect of energetic materials via advanced isoconversional decomposition kinetics. J Therm Anal Calorim. 2018;133(1):737–44.
Pouretedal HR, Damiri S, Sharifi A. Study of triplet kinetic of thermal decomposition reaction and sensitivity to impact and electrostatic discharge of HMX polymorphs. J Therm Anal Calorim. 2020;139(1):545–53.
Pitchimani R, Hope-Weeks LJ, Zhang G, Weeks BL. Effect of impurity doping on the morphology of pentaerythritol tetranitrate crystals. J Energ Mater. 2007;25:203–12.
Maridha S, Weeks BL. Effect of Zn doping on the sublimation rate of pentaerythritol tetranitrate using atomic force microscopy. Scanning. 2009;31:181–7.
Xu J, Liu Y, Zhang H, Sun J. Influence and action mechanism of additives on heat-induced polymorphic transformation of HNIW. Chin J Energ Mater. 2018;26(08):645–52.
Zhang G, Bhattachacharia SK, Weeks BL. Effect of Zinc doping on pentaerythritol tetranitrate single crystals. Cryst Res Technol. 2010;45(7):732–6.
Pitchimani R, Zheng W, Simon SL, Hope-Weeks LJ, Burnham AK, Weeks BL. Thermodynamic analysis of pure and impurity doped pentaeryththritol tetranitrate crystals grown at room temperature. J Therm Anal Calorim. 2007;89(2):475–8.
Pitchimani R, Burnham AK, Weeks BL. Quantitative thermodynamic analysis of sublimation rates using an atomic force microscope. J Phys Chem B. 2007;111(31):9182–5.
Sudhakar AO, Mathew S. Thermal behaviour of CuO doped phase-stabilized ammonium nitrate. Thermochim Acta. 2006;451:5–9.
Gao H, Zhang Q, Shreeve JM. Fused heterocycle-based energetic materials (2012–2019). J Mater Chem A. 2020;8(8):4193–216.
Zhang S, Gao Z, Lan D, Jia Q, Liu N, Zhang J, Kou K. Recent advances in synthesis and properties of nitrated-pyrazoles based energetic compounds. Molecules. 2020;25(15):3475.
Gong F, Yang Z, Qian W, Liu Y, Zhang J, Ding N, Lin C, Zeng C, Yan Q. β→δ polymorphic transformation: Kinetics for inhibited polymorphic transition of HMX crystal after strong surface confinement. J Phys Chem C. 2019;123(17):11011–9.
Tao J, Wang X. Crystal structure and morphology of β-HMX in acetone: a molecular dynamic simulation and experimental study. J Chem Sci. 2017;129(4):495–503.
Li J, Jin S, Lan G, Xu L, Chen S, Li L. The effect of solution conditions on the crystal morphology of β-HMX by molecular dynamics simulations. J Cryst Growth. 2019;507(1):38–45.
Pinheiro GFM, Loureno VL, Iha K. Influence of the heating rate in the thermal decomposition of HMX. J Therm Anal Calorim. 2002;67(2):445–52.
Singh A, Kumar R, Soni PK, Singh V. Compatibility and thermal decomposition kinetics between HMX and some polyester-based polyurethanes. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09377-5.
Mittemeijer EJ, Welzel U. Modern diffraction methods. New York: Wiley; 2013.
Young RA. The Rietveld Method. Oxford: Oxford University Press; 2002.
Liu Y, Li S, Wang Z, Xu J, Sun J, Huang H. Thermally induced polymorphic transformation of hexanitrohexaazaisowurtzitane (HNIW) investigated by in-situ X-ray powder diffraction. Cent Eur J Energ Mater. 2016;13(4):1023–37.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21805259 and 21975234).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, J., Cheng, K., Zhang, H. et al. Effect of metal ion impurities on the crystallization and thermal properties of HMX. J Therm Anal Calorim 147, 6109–6118 (2022). https://doi.org/10.1007/s10973-021-10850-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10850-y