Abstract
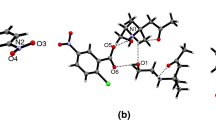
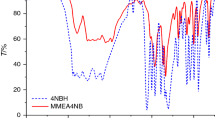
Two new multicomponent crystal forms based on 4-nitrobenzoic acid-diethanolamine system, 1:1 molecular salt and 1:1:1 salt cocrystal were synthesized in the same conditions but different solvents and analyzed by complementary experimental techniques such as single-crystal X-ray diffraction, infrared absorption spectroscopy, thermogravimetric and kinetic analyses. The compounds differ from each other by the inclusion of 4-nitrobenzoic acid molecule in salt cocrystal and by the way of packaging of the components in crystals, as well as by the mode of connecting of organic cations with anions. Thermogravimetric analysis and kinetic study complete the structural study of the new obtained multicomponent crystals, providing additional information on their thermal stability and degradation process. The packing and inter-/intramolecular differences justify the melting points and enthalpy of fusions for the compounds studied. A good correlation of the activation energy values obtained by four different isothermal and non-isothermal isoconversional methods was observed. In both cases, the additional acid molecule in salt cocrystal decreases the activation energy values compared to salt, which can be related to a slower thermal degradation. Comparative investigation on photoluminescence properties was also discussed.






Similar content being viewed by others
References
Aakeröy CB, Fasulo ME, Desper J. Cocrystal or salt: does it really matter? Mol Pharm. 2007;4:317–22.
Guo W, Du S, Lin Y, Lu B, Yang C, Wang J, Zeng Y. Structural and computational insights into the enhanced solubility of dipfluzine by complexation: salt and salt-cocrystal. New J Chem. 2018;42:15068–78.
Grothe E, Meekes H, Vlieg E, ter Horst JH, de Gelder R. Solvates, salts, and cocrystals: a proposal for a feasible classification system. Cryst Growth Des. 2016;16:3237–43.
Braga D, Grepioni F, Maini L, Prosperi S, Gobetto R, Chierotti MR. From unexpected reactions to a new family of ionic co-crystals: the case of barbituric acid with alkali bromides and caesium iodide. Chem Commun. 2010;46:7715–7.
Braga D, Grepioni F, Shemchuk O. Organic-inorganic ionic co-crystals: a new class of multipurpose compounds. CrystEngComm. 2018;20:2212–20.
Lou B, Perumalla SR, Sun CC. Significant expansion of the solid state landscape of salicylic acid based on charge-assisted hydrogen bonding interactions. Cryst Growth Des. 2014;15:24–8.
Lou B, Perumalla SR, Sun CC. From molecular salt to pseudo CAB cocrystal: expanding solid-state landscape of carboxylic acids based on charge-assisted COOH/COO− hydrogen bonds. J Mol Struct. 2015;1099:516–22.
Li D, Kong M, Li J, Dengab Z, Zhang H. Amine-carboxylate supramolecular synthon in pharmaceutical cocrystals. CrystEngComm. 2018;20:5112–8.
Oruganti M, Nechipadappu SK, Khade PA, Trivedi DR. Solid-state versatility of the molecular salts/cocrystals of 2-chloro-4-nitrobenzoic acid: a case study on halogen bonds. ACS Omega. 2017;2:7146–62.
Cruz-Cabeza AJ. Acid–base crystalline complexes and the pKa rule. CrystEngComm. 2012;14:6362–5.
Cox BG. Acids and bases. Solvent effects on acid-base strength. Oxford: Oxford University Press; 2013.
Croitor L, Petric M, Vlase G, Vlase T, Siminel AV, Bourosh P, Crisan M. The solvent effect in obtaining of acid-base multicomponent systems: thermal, structural and luminescence study. J Therm Anal Calorim. 2020;141:973–9.
Ganduri R, Cherukuvada S, Guru Row TN. Multicomponent adducts of pyridoxine: an evaluation of the formation of eutectics and molecular salts. Cryst Growth Des. 2015;15:3474–80.
Selvakumar E, Anandha Babu G, Ramasamy P, Rajnikant, Murugesan V, Chandramohan A. Synthesis, growth and spectroscopic investigation of an organic molecular charge transfer crystal: 8-hydroxy quinolinium 4-nitrobenzoate 4-nitrobenzoic acid. Spectrochim Acta A Mol Biomol Spectrosc. 2014;117:259–63.
Saunders LK, Nowell H, Spencer HCE, Hatcher LE, Shepherd HJ, Thomas LH, Jones CL, Teat SJ, Raithby PR, Wilson CC. Tuning charge-assisted and weak hydrogen bonds in molecular complexes of the proton sponge DMAN by acid co-former substitution. CrystEngComm. 2018;20:3074–83.
Smith G, Lynch DE. Cyclic heterotetrameric and low-dimensional hydrogen-bonded polymeric structures in the morpholinium salts of ring-substituted benzoic acid analogues. Acta Crystallogr C Struct Chem. 2016;72:105–11.
Croitor L, Crisan M, Vlase G, Vlase T, Bodnarescu F, Sumalan R, Petric M, Siminel AV, Bourosh P. Advances in new multicomponent crystal system: structure, thermal kinetic analysis, photoluminescent, and biological activity investigations. J Therm Anal Calorim. 2020;142:191–201.
Croitor L, Petric MF, Szerb EI, Vlase G, Bourosh P, Chumakov Y, Crisan M. Role of 4-nitrobenzoic acid polymorphs in the crystallization process of the organic acid-base multicomponent system. CrystEngComm. 2019;21:6038–47.
Chumakov YM, Simonov Y, Grozav M, Crisan ME, Bocelli G, Yakovenko AA, Lyubetsky D. Hydrogen-bonding network in the organic salts of 4-nitrobenzoic acid. Cent Eur J Chem. 2006;4:458–75.
Crisan ME, Bourosh P, Chumakov YM, Petric M, Ilia G. Supramolecular assembly and Ab initio quantum chemical calculations of 2-hydroxyethylammonium salts of para-substituted benzoic acids. Cryst Growth Des. 2013;13:143–54.
Crisan M, Halip L, Bourosh P, Chicu SA, Chumakov Y. Synthesis, structure and toxicity evaluation of ethanolamine nitro/chloronitrobenzoates: a combined experimental and theoretical study. Chem Cent J. 2017;11:129–39.
Crisan M, Vlase G, Plesu N, Petric M, Croitor L, Kravtsov V, Chumakov Y, Bouros P, Vlase T. Ethylethanolammonium 4-nitrobenzoate: synthesis, structural characterization, thermal analysis, non-isothermal kinetic investigations and corrosion inhibitor efficiency. J Therm Anal Calorim. 2018;134:343–52.
Crisan M, Vlase G, Szerb EI, Vlase T. Thermal and kinetics studies of primary, secondary and tertiary alkanolammonium salts of 4-nitrobenzoic acid. J Therm Anal Calorim. 2018;132:1409–18.
Sumalan RL, Croitor L, Petric M, Radulov I, Bourosh P, Sumalan RM, Crisan M. p-Aminobenzoate organic salts as potential plant growth regulators for tomatoes. Molecules. 2020;25:1635–49.
Grozav M, Neamtiu I, Dorosencu M, Laichici M, Mercea M. The synthesis of some ammonium salts of benzoic acids with ethanolamine, possible plant growth stimulators. Rev Chim. 2003;54:287–8.
Gorobet A, Crisan M, Petric M, Bourosh P, Croitor L. Structural study of Ca(II) coordination compound with triethanolamine and 4-nitrobenzoic acid. Rev Roum Chim. 2018;63:1175–9.
Sheldrick GM. Crystal structure refinement with SHELXL. Acta Cryst. 2015;C71:3–8.
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, Streek J. Mercury: visualization and analysis of crystal structures. J Appl Crystallogr. 2006;39:453–7.
Groom CR, Bruno IJ, Lightfoot MP, Ward SC. The Cambridge structural database. Acta Cryst B. 2016;B72:171–9.
Pavia DL, Lampman GM, Kriz GS. Introduction to spectroscopy: a guide for students of organic chemistry. 3rd ed. Andover: Tomson Learning; 2001.
Friedman HL. Kinetics of thermal degradation of char-foaming plastics from thermogravimetry: application to a phenolic resin. J Polym Sci. 1965;6C:183–95.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. Polym Lett. 1966;4:323–8.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Akahira T, Sunose T. Joint convention of four electrical institutes. Research Report Chiba Institute of Technology. Sci Technol. 1971;16:22–31.
Ledeti I, Murariu M, Vlase G, Vlase T, Doca N, Ledeti A, Suta LM, Olariu T. Investigation of thermal-induced decomposition of iodoform. J Therm Anal Calorim. 2017;127:565–70.
Doca N, Vlase G, Vlase T, Perţa M, Ilia G, Plesu N. TG, EGA and kinetic study by non-isothermal decomposition of a polyaniline with different dispersion degree. J Therm Anal Calorim. 2009;97(2):479–84.
Vlase T, Vlase G, Doca N, Iliescu S, Ilia G. Thermo-oxidative degradation of polymers containing phosphorus in the main chain. High Perform Polym. 2010;22(7):863–75.
Bolcu C, Vlase G, Vlase T, Albu P, Doca N, Şisu E. Thermal behavior of some polyurethanes reticulated by aminated maltose. J Therm Anal Calorim. 2013;113(3):1409–14.
Serra R, Nomen R, Sempere J. The non-parametric kinetics. A new method for the kinetic study of thermoanalytical data. J Therm Anal Calorim. 1998;52:933–43.
Serra R, Sempere J, Nomen R. A new method for the kinetic study of thermoanalytical data: the non-parametric kinetics method. Thermochim Acta. 1998;316:37–45.
Vlase G, Bolcu C, Modra D, Budiul MM, Ledeți I, Albu P, Vlase T. Thermal behavior of phthalic anhydride-based polyesters. J Therm Anal Calorim. 2016;126:287–92.
Ceban I, Blajovan R, Vlase G, Albu P, Koppandi O, Vlase T. Thermoanalytical measurements conducted on repaglinide to estimate the kinetic triplet followed by compatibility studies between the antidiabetic agent and various excipients. J Therm Anal Calorim. 2016;126:195–204.
Patrutescu C, Vlase G, Turcus V, Ardelean D, Vlase T, Albu P. TG/DTG/DTA data used for determining the kinetic parameters of the thermal degradation process of an immunosuppressive agent: mycophenolate mofetil. J Therm Anal Calorim. 2015;121(3):983–8.
Ŝestak J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12.
Losev EA, Boldyreva EV. A salt or a co-crystal—when crystallization protocol matters. CrystEngComm. 2018;20:2299–305.
Losev EA, Boldyreva EV. The effect of amino acid backbone length on molecular packing: crystalline tartrates of glycine, β-alanine, γ-aminobutyric acid (GABA) and DL-α-aminobutyric acid (AABA). Acta Crystallogr C Struct Chem. 2018;74:177–85.
Acknowledgements
This work was partially supported by Program 2, Project 2.1 of the “Coriolan Drăgulescu” Institute of Chemistry and by the project ANCD 20.80009.5007.15 of the Institute of Applied Physics.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Crisan, M., Petric, M., Vlase, G. et al. Organic salt versus salt cocrystal: thermal behavior, structural and photoluminescence investigations. J Therm Anal Calorim 147, 1203–1213 (2022). https://doi.org/10.1007/s10973-020-10438-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10438-y