Abstract
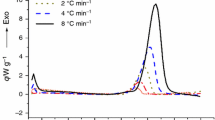
This paper presents the results obtained after the investigation of thermal-induced decomposition of tri-iodo-substituted methane (iodoform), under non-isothermal conditions in dynamic oxidative atmosphere (air flow). Iodoform was obtained in our laboratory through haloform reaction, namely the halogenation of dimethyl ketone in the presence of a base, and later purified according to literature. The kinetic study for the thermodegradation was carried out by processing the data by Friedman, Flynn–Wall–Ozawa, Kissinger–Akahira–Sunose and modified nonparametric kinetic (NPK) methods. All the results obtained by the employment of isoconversional methods are in good agreement; the mean apparent activation energy was around 330 kJ mol−1, by all three methods. However, since variation larger than 10 % of E a versus α was noticed, the NPK method was employed, and the separation of physical versus chemical transformations of the sample was possible. NPK method suggested that the degradation of iodoform occur in two parallel processes, the main step being a chemical degradation (n = 1/3), while the parallel process is a physical–chemical one (n = 3/5; m = 1/3).







Similar content being viewed by others
References
Hill B. On the therapeutic use of iodoform. Brit Med J. 1878;1:127.
Freedman M, Stassen LFA. Commonly used topical oral wound dressing materials in dental and surgical practice–a literature review. J Irish Dent Assoc. 2013;59(4):190–5.
Lyday PA. Iodine and iodine compounds, Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH; 2005.
Lin BC, Zhao YM, Yang J, Wang WJ, Ge LH. Effects of zinc oxide-eugenol and calcium hydroxide/iodoform on delaying root resorption in primary molars without successors. Dent Mater J. 2014;33(4):471–5.
Kuga MC, Faria G, Só MV, Keine KC, dos Santos AD, Duarte MAH, Kopper PMP. The impact of the addition of iodoform on the physicochemical properties of an epoxy-based endodontic sealer. J Appl Oral Sci. 2014;22(2):125–30.
Ashraf N, Capper R. Should we still be using bismuth iodoform paraffin paste-impregnated gauze as an ear canal dressing following ear surgery? Clin Otolaryngol. 2013;38(4):357–60.
Ladak A, Agrawal S. Bismuth iodoform paraffin paste: a review. J Laryngol Otol. 2013;127(5):531–41.
Petel R, Moskovitz M, Tickotsky N, Halabi A, Goldstein J, Houri-Haddad Y. Cytotoxicity and proliferative effects of Iodoform-containing root canal-filling material on RAW 264.7 macrophage and RKO epithelial cell lines. Arch Oral Biol. 2013;58(1):75–81.
Abraham A, Zhang SS, Aly Y, Schoenitz M, Dreizin EL. Aluminum-iodoform composite reactive material. Adv Eng Mater. 2014;16(7):909–17.
Gu JM, Yan XH, Fu ZF, Yang WT, Shi Y. Iodoform-mediated free radical emulsion polymerization of chloroprene. J Appl Polym Sci. 2013;128(4):2291–6.
Bertolotti F, Gervasio G. Crystal structure of iodoform at 106 K and of the adduct CHI3 center dot 3(C9H7N). Iodoform as a building block of co-crystals. J Mol Struct. 2013;1036:305–10.
Bielinski DM, Slusarski L, Glab P. Modification of rubber by iodoform. J Appl Polym Sci. 2007;105(1):177–89.
Fulias A, Popoiu C, Vlase G, Vlase T, Onetiu D, Savoiu G, Simu G, Patrutescu C, Ilia G, Ledeti I. Thermoanalytical and spectroscopic study on methotrexate—active substance and tablet. Dig J Nanomater Bios. 2014;9:93–8.
Ledeti I, Vlase G, Vlase T, Fulias A. Kinetic analysis of solid-state degradation of pure pravastatin versus pharmaceutical formulation. J Therm Anal Calorim. 2015;121(3):1103–10.
Anghel M, Vlase G, Bilanin M, Vlase T, Albu P, Fulias A, Tolan I, Doca N. Comparative study on the thermal behavior of two similar triterpenes from birch. J Therm Anal Calorim. 2013;113(3):1379–85.
Fulias A, Vlase G, Grigorie C, Ledeti I, Albu P, Bilanin M, Vlase T. Thermal behaviour studies of procaine and benzocaine: Part 1. Kinetic analysis of the active substances under non-isothermal conditions. J Therm Anal Calorim. 2013;113(1):265–71.
Ledeti I, Fulias A, Vlase G, Vlase T, Bercean V, Doca N. Thermal behaviour and kinetic study of some triazoles as potential anti-inflammatory agents. J Therm Anal Calorim. 2013;114:1295–305.
Fulias A, Ledeti I, Vlase G, Vlase T. Physico-chemical solid-state characterization of pharmaceutical pyrazolones: an unexpected thermal behaviour. J Pharm Biomed. 2013;81–82:44–9.
Ivan C, Suta LM, Olariu T, Ledeti I, Vlase G, Vlase T, Olariu S, Matusz P, Fulias A. Preliminary kinetic study for heterogenous degradation of cholesterol-containing human biliary stones. Rev Chim Bucharest. 2015;66(8):1253–5.
Pupca G, Bucuras V, Vlase G, Vlase T, Fulias A, Ledeti I. Solid-state analysis of some urinary calculi. Rev Chim Bucharest. 2014;65(9):1058–62.
Ivan C, Ledeti I, Vlase G, Vlase T, Fulias A, Olariu S. Study of solid-state behaviour of some human gallstones. Rev Chim Bucharest. 2015;65(2):265–70.
Fuson RC, Tullock CW. The Haloform Reaction. XIV. An improved iodoform test. J Am Chem Soc. 1934;56(7):1638–40.
Rybinski P, Janowska G, Antkowicz W, Krauze S. Thermal stability and flammability of butadiene-acrylonitrile rubber cross-linked with iodoform. J Therm Anal Calorim. 2005;81(1):9–13.
Janowska G, Kucharska A. The influence of the method of butadiene rubbers cross-linking on their thermal properties. J Therm Anal Calorim. 2009;96(2):561–5.
Rybinski P, Janowska G. Effect of the spatial network structure and cross-link density of diene rubbers on their thermal stability and fire hazard. J Therm Anal Calorim. 2014;117(1):377–86.
Rybinski P, Kucharska-Jastrzaek A, Janowska G. Thermal properties of diene elastomers. Polym Sci Ser B. 2014;56(4):477–86.
Chakrabartty SK. Alkaline hypohalite oxidations. In: Trahanovsky WH, editor. Oxidation in organic chemistry. New York: Academic Press; 1978. pp. 343–70.
Friedman HL. Kinetics of thermal degradation of char-foaming plastics from thermogravimetry: application to a phenolic resin. J Polym Sci. 1965;6C:183–95.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. Polym Lett. 1966;4:323–8.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Akahira T, Sunose T. Joint convention of four electrical institutes. Research Report Chiba Institute of Technology. Sci Technol. 1971;16:22–31.
Serra R, Nomen R, Sempere J. The non-parametric kinetics. A new method for the kinetic study of thermoanalytical data. J Therm Anal Calorim. 1998;52:933–43.
Serra R, Sempere J, Nomen R. A new method for the kinetic study of thermoanalytical data: the non-parametric kinetics method. Thermochim Acta. 1998;316:37–45.
Vlase T, Vlase G, Doca N, Bolcu C. Processing of non-isothermal TG data. Comparative kinetic analysis with NPK method. J Therm Anal Calorim. 2005;80:59–64.
Vlase T, Vlase G, Doca N, Ilia G, Fulias A. Coupled thermogravimetric-IR techniques and kinetic analysis by non-isothermal decomposition of Cd2+ and Co2+ vinyl-phosphonates. J Therm Anal Calorim. 2009;97:467–72.
Bodescu AM, Sirghie C, Vlase T, Doca N. Comparative kinetics studies of thermal decomposition of kalium, respectively natrium oxalato-oxo-diperoxo molibdate. J Therm Anal Calorim. 2013;113(3):1431–5.
Šestak J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ionuţ Ledeţi and Marius Murariu have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Ledeţi, I., Murariu, M., Vlase, G. et al. Investigation of thermal-induced decomposition of iodoform. J Therm Anal Calorim 127, 565–570 (2017). https://doi.org/10.1007/s10973-016-5368-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5368-z