Abstract
The work presents the results of thermodynamic analysis of two medium manganese steels with different Mn contents. The steels containing 3.1 and 3.6% of manganese were subjected to theoretical thermodynamic calculations using MUCG83 software and dilatometric experiments. The steels were heat-treated in two different isothermal holding temperatures of 400 and 350 °C for 15 min. The bainite transformation kinetics at different temperatures for different manganese contents was investigated. In the steel including 3.1% Mn, a complete transformation was obtained. The results indicated a strong influence of the holding temperature on the kinetics of bainitic transformation. It was related to the driving force of this process. When the manganese content was increased by 0.5%, an incomplete bainite transformation occurred. The microstructure investigations after heat treatment were performed using light and scanning electron microscopy. The XRD analysis to determine retained austenite amount and its carbon enrichment was performed. The microstructure of 3MnNb steel consisted of bainite and retained austenite with filmlike and blocky morphologies. The steel with the higher Mn content contained also fresh martensite for both isothermal holding temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of new materials for automotive industry strongly affects various investigations conducted around the world. The design process needs to take into account safety and environmental aspects of car body production. One of the most important factors in car body manufacture is the material price. Medium-Mn steels are dedicated to the automotive sector due to their multiphase structure generating very beneficial properties. These steels are designed to compromise between cost and mechanical properties. Medium-Mn steels should have higher mechanical properties than first generation AHSS but be cheaper than second generation of AHSS (advanced high-strength steels) [1,2,3]. The main structural constituent that gives these steels good formability is retained austenite. This phase undergoes strain-induced martensitic transformation. A local increase in strength accompanying this transformation allows prolonging deformation, which finally leads to higher formability and mechanical properties [4,5,6]. These aspects allow to implement such steels for cold-rolled steel coils for the automotive application. This approach ensures the formation of retained austenite in a bainite matrix, which can enhance the formability of the steel, increasing the range of possible product applications (especially with the complex shape). That’s why the knowledge on phase transformation kinetics of various phases is very important.
To determine phase transitions in metallic materials during heating and cooling a thermal analysis is used. This method gives detailed information on a phase transformation kinetics rate and critical temperatures of different phases in the liquid and solid states [7, 8]. One of them is dilatometry combining a linear thermal expansion principle with phase transformations occurring during heat treatment. This method can be applied during continuous cooling or under conditions of isothermal treatment. For the isothermal bainite transformation, it is possible to determine its rate and amount of a formed phase at different temperatures or incubation times. At the same time, austenite stability during cooling to room temperature after the isothermal step may be examined.
Medium-Mn steels are usually investigated in terms of austenite stability upon continuous cooling from an intercritical annealing step performed in an austenite–ferrite region [9, 10]. A few CCT or TTT diagrams for medium-Mn steels can be also found in the literature [11]. However, so far there are no systematic studies on transformation kinetics under conditions of isothermal bainitic transformation. Therefore, the study aims at providing some computational and experimental data on the isothermal bainitic transformation kinetics in two lean medium-Mn steels. The effects of manganese content, isothermal holding temperature and prior martensite presence on the bainite transformation kinetics are addressed.
Experimental
For the analysis of bainitic transformation kinetics and austenite stability, two different medium manganese steels were investigated. The chemical composition of the steels is presented in Table 1.
The chemical compositions are very similar except a Mn content, which is a basis for steel coding: 3MnNb and 4MnNb. Both steels include the increased manganese content to stabilize retained austenite. A high aluminum content prevents carbide formation during bainitic transformation leading to higher enrichment of the austenite in carbon.
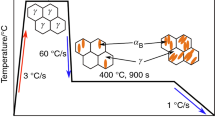
The thermodynamic analysis was performed using MUCG database ver. 83 software [12] to determine the effect of chemical composition (especially a Mn content) on the phase transformation kinetics for both steels. The heat treatment performed in the dilatometer is presented in Fig. 1.
The samples were heated up to 1100 °C at a rate of 3 °C s−1 and kept in this temperature for 300 s to homogenize austenite microstructure. After this step, the material was cooled at a rate of 60 °C s−1 to selected isothermal holding temperatures: 400 and 350 °C. The duration of the isothermal step was 900 s. After this step, the samples were cooled down to room temperature at a rate of 1 °C s−1. These parameters were selected according to theoretical calculations carried out using JMatPro software in another study [13]. According to this study, 60 °C s−1 ensures no transformation during cooling to isothermal holding temperature. The 400 and 350 °C were selected to ensure a bainite formation during the isothermal holding. However, in case of 3MnNb 350 °C treatment, the formation of some martensite is expected (holding below Ms temperature).
All heat treatments were performed on 4-mm-diameter and 10-mm-length cylinders in a BAHR 805A high-resolution dilatometer. Cooling is applied by blowing helium to the sample, which was used as a quenching gas and the temperature was controlled by a type K thermocouple welded to the central part of the sample surface. The dilatometry test was performed using a specific module, equipped with fused silica push-rods to measure the longitudinal changes during different stages of the heat treatments. From the obtained data, critical transformation temperatures, Ac1, Ac3 and Ms, and the kinetics of bainitic transformation were determined. The specimens were sectioned and polished following conventional metallographic techniques, and the microstructure was revealed by etching with Nital 2%.
X-ray diffraction measurements were carried out with a Bruker AXS D8 diffractometer equipped with a Co X-ray tube. A current of 30 mA and a voltage of 40 kV were employed as tube settings. XRD data were collected over a 2θ range of 35°–135° with a step size of 0.01°. To this end, samples were prepared following the standard metallographic procedures finishing with 1 μm diamond paste.
Results and discussion
Theoretical calculations
A first step was theoretical calculations for investigated steels. First, the T0 curves (Fig. 2) and driving force for the bainite transformation (Fig. 3) were calculated using a MUCG software [12]. It was done to determine the effects of temperature and manganese content on a driving force of bainite transformation. According to the presented curves, the isothermal holding temperature has an impact on the driving force for bainite transformation. Lowering temperature of this step from 400 to 350 °C leads to the higher driving force and resulting increase in the amount of bainitic phase. Together with the higher amount of bainite, the carbon enrichment of austenite increases too. This is because bainite dissolves lower amount of carbon compared to austenite. This excess carbon diffuses to austenite increasing its stability. On the other hand, the increase in manganese content gives an opposite result. When manganese content is increased, the T0 curve shifts to the left. This means the lower amount of bainite and corresponding smaller carbon enrichment of the austenite. The calculations of the driving force show that a decrease in temperature by 50 °C increases the driving force by 220 J mol−1. In case of manganese, its 0.5% increase reduces the driving force by 25 J mol−1. These results indicate that temperature has a higher impact on the driving force of bainite transformation than a manganese content. However, in case of the analysis for 3MnNb steel at 350 °C, a martensite transformation needs to be taken into account. On the basis of the martensite start temperature (Ms) calculation, it was determined that Ms for this steel is ~ 400 °C. It means that during cooling to isothermal holding temperature of 350 °C, some martensite is formed. This will affect the bainite transformation kinetics [14].
To determine if the calculated carbon enrichment is enough to stabilize austenite to room temperature, the martensite start temperature was determined as a function of carbon content [13]. These results for both steels are presented in Table 2. According to the presented results, one can see that obtained carbon enrichment is not enough to stabilize austenite to room temperature in both cases. The calculations indicate that for 3MnNb steel, the carbon enrichment of austenite is ca. 1.1% and for 4MnNb ~ 1%. Therefore, some fraction of “fresh” martensite could be formed during cooling to room temperature.
Dilatometric analysis
After thermodynamic calculations, the dilatometric study was carried out. The tests were performed according to Fig. 1. A first step was to analyze dilatometric curves during heating to determine the repeatability of the tests. Figure 4 presents the curves registered for all heat treatment variants.
According to the dilatometric curves, some differences can be seen. The difference between 3MnNb and 4MnNb steels is due to a manganese content. The differences in 4MnNb steel curves may indicate some segregation of alloying elements. This means that some issues may occur during bainite transformation. Additionally, the Ac1 and Ac3 temperatures for both steels were determined. The austenite transformation for 3MnNb steel starts at 715 °C and finishes at 1015 °C. The 4MnNb steel has a similar starting temperature of 705 °C but a lower finish transformation temperature of 970 °C. This change in finish temperature is caused by higher manganese content in the 4MnNb steel. The manganese is an austenite stabilizer [6]. That is why the decrease in Ac3 temperature can be seen [15]. According to the registered results, the selected austenitization temperature was correct.
During cooling to an isothermal holding temperature, only 3MnNb steel at 350 °C has a different curve compared to the others (Fig. 5). In this case, a small increase in relative change of length can be seen. This corresponds to a volume increase in samples indicating transformation of some austenite fraction into martensite. This results from the fact that the isothermal holding temperature of 350 °C is lower than Ms temperature of 3MnNb steel. The other curves do not indicate any martensite transformation. According to Guo et al. [14], prior martensite formed before an isothermal treatment step has a high impact on the bainitic transformation kinetics. The prior martensite locally deforms the austenite increasing its dislocation density. This changes conditions for nucleation and growth of bainite and decreases the energy required for heterogeneous bainite nucleation. Hence, the higher amount of nucleation sites is present, which influence the incubation and bainitic transformation times. Similar results were obtained by Navarro-Lopez et al. [15]. They also showed that the formation of prior martensite strongly affects the bainite transformation kinetics. When the amount of prior martensite increased, the rate of transformation increased too.
The analysis of isothermal holding step was performed for different temperatures and manganese contents. The dilatometric curves registered during the isothermal step for 3MnNb steel are presented in Fig. 6a.
First thing that may be seen is that at 400 °C, a higher signal from bainitic transformation was obtained. The reason for this may be a smaller amount of austenite transformed into bainite at 350 °C due to martensite formation. In the research of Navarro-Lopez et al. [15], the authors showed that the formation of prior martensite decreased the amount of bainite created during the isothermal step. Moreover, the prior martensite undergoes tempering resulting in carbon diffusion from martensite to austenite. The diffusion led to contraction, which affects a level of signal at 350 °C. In both cases, the transformation was finished after 10 min. This means that a carbon enrichment of the austenite was high enough to be in equilibrium with bainite (amount of carbon in T0 curve). No further carbon enrichment of austenite is possible for this steel. Moreover, a difference in bainitic transformation rate can be seen. The 350 °C sample has the higher transformation rate (Fig. 6b) compared to 400 °C, especially at the beginning of bainite transformation. The reason for this fact is mentioned prior martensite created before the isothermal step. The martensite locally deforms the austenite resulting in the higher amount of nucleation places for bainite transformation [14, 16]. This accelerates the rate of bainite formation at this temperature. There is no incubation time for both isothermal holding temperatures. This is because of high aluminum content of the steel (1.6%). Because Al is an α phase stabilizer, it increases the driving force for bainite transformation as presented in Fig. 7. The figure presents the results of Al addition on a driving force of bainite transformation for different Al contents. The calculations included three aluminum contents: 0, 0.8 and 1.6%. The high aluminum content in steel increases the driving force, which accelerates the transformation significantly. The difference in driving force between 0 and 1.6% of Al is 306 J mol−1. This is the reason for a lack of incubation time for both samples. A similar conclusion reached Tian et al. [17], who investigated the influence of Cr and Al on the bainite transformation. The bainite transformation in their case was faster in steel with the addition of aluminum. Similarly, according to Caballero and Bhadeshia [18], aluminum increases the driving force for the transformation of austenite into bainite, which leads to a higher transformation rate.
The last step of the heat treatment after isothermal holding was cooling to room temperature. Results are presented in Fig. 8. In both cases, during cooling to room temperature no fresh martensite was formed. This means that the carbon enrichment of the austenite was high enough to stabilize it to room temperature. It is true even though theoretical calculations show that the level of enrichment should not be enough.
The results for 4MnNb isothermal holding step are presented in Fig. 9. The dilatometric curves show a bainite transformation kinetics at 400 and 350 °C. One can see an incomplete bainite transformation for both temperatures. In both cases, the bainite transformation is not finished within 15 min. It means that γ to α equilibrium was not obtained. Therefore, the carbon enrichment of the austenite is not sufficient and some fresh martensite should be expected during cooling to room temperature.
In case of 400 °C, an incubation time is visible. It means that manganese has a strong impact on the bainite transformation kinetics. Manganese influences the transformation by shifting its region in a TTT curve to longer time. That’s why an incubation and incomplete transformation take place. Similar results are presented in the work of Guo et al. [19], which reported that manganese retards the bainite transformation. In their case, an increase of 0.5% Mn led to two times longer incubation time and a lower transformation rate. According to Farahani et al. [20], who carried out theoretical calculations of Si and Mn effects on a bainite amount, manganese decreases the amount of bainite that can be obtained. The reason for this is a high value of dissipation of Gibbs energy caused by interfacial diffusion of Mn. This is in accordance with the presented results. The 4MnNb steel needs longer time to complete bainite transformation, and the transformation rate is lower compared to the 3MnNb steel. When the temperature was decreased to 350 °C, the driving force for the transformation increased. The result of this is a higher dilatometric signal during this step. The higher driving force means more bainite, which in turn causes a higher carbon enrichment of the austenite. In this case, a higher stability of austenite should be expected (a lower amount of fresh martensite during cooling to room temperature).
When looking at Fig. 9, one can see a contrary conclusion. According to the theory, lowering the isothermal holding temperature should increase the incubation time and signal from the bainite transformation. The higher signal is present but the incubation is not observed. The explanation is a small amount of martensitic phase before the isothermal step. The amount is so low that there is no visible signal on a dilatometric curve during cooling to isothermal holding. However, the amount is enough to accelerate the kinetics, which is visible at the beginning of bainite transformation. The formation of martensite could occur because of segregation issues detected by dilatometry. According to Grajcar et al. [21], some segregation may occur in medium-Mn steels. Based on this research, a calculation of martensite start temperature was carried out to determine the possible difference in Ms temperature of these steels. According to this calculation, the maximum difference between lowest and highest Ms temperature was 50 °C. This could explain the theory of small amount of martensite present before the isothermal step, which accelerates the bainitic transformation.
During cooling to room temperature, the transformation of fresh martensite could be detected (Fig. 10). The bigger signal at 400 °C corresponded to a lower amount of bainite formed during the isothermal step (Fig. 9). This leads to lower carbon enrichment and corresponding lower thermal stability of austenite. This is indicated also by the Ms temperature. For the lower thermal stability, a higher martensite start temperature is expected. When the temperature was lowered, the amount of bainite increased. An increase in carbon content of the austenite (higher thermal stability) directly decreases the Ms temperature. According to this result, the selected time was too short to fully stabilize the austenite at room temperature.
Microstructure investigation
After the dilatometric study, the microstructure analysis was carried out. The microstructure presented in Fig. 11 shows the occurrence of segregation bands for all samples. These results are in accordance with the dilatometric analysis during heating to austenitization temperature. In case of 4MnNb steel, this can prove that there is a possibility of locally higher Ms temperature. Similar conclusion was reported in [15]. The authors stated that banding occurs due to manganese segregation, which created high-Mn and low-Mn regions. These areas have different local Ms temperatures and may form small amounts of martensite, which influence the bainitic transformation kinetics as explained earlier.
Figures 12 and 13 show microstructures of 3MnNb steel processed at different temperatures of isothermal holding. At 400 °C, a fine-grained microstructure composed of bainite and retained austenite can be seen in Fig. 12a. A more detailed micrograph taken using scanning electron microscopy (Fig. 12b) shows that a small amount of martensitic–austenitic islands (MA) is also present. The lack of detection of martensitic transformation by a dilatometer indicates its small amount. The retained austenite in the microstructure takes shape of thin layers between bainitic laths. This morphology positively influences its thermal stability. According to Xiaochuan et al. [22], this kind of morphology strongly stabilizes retained austenite. In this work, two types of retained austenite were present. The first type was characterized by a blocky microstructure. In the second case, the film shape was visible. The filmlike retained austenite had approximately two times lower carbon content, yet it was stable to room temperature. The authors proposed two explanations for this phenomenon. The first one was the presence of martensite near retained austenite. This hard phase due to its high strength inhibits further martensitic transformation blocking the possibility of austenite expansion (this is a kind of physical stabilization of austenite). The other possibility was exerted by residual stresses, which create hydrostatic pressure on the retained austenite. The hydrostatic pressure prevents expansion, thus hindering martensitic transformation. In the current work, the second theory is more presumable because the higher transformation kinetics leads to higher residual stresses [20].
At 350 °C, the microstructure is composed of bainite, some amount of tempered martensite and retained austenite (Fig. 13a). In case of bainite plates formed at 350 °C, their bigger thickness and higher amount can be observed compared to 400 °C. These differences result from the increased driving force of bainitic transformation at this temperature. A higher magnification analysis (Fig. 13b) shows the higher amount of bainite and its bigger plate thickness. Moreover, the bainite has two types. The major one is lath bainite and the second one is coalesced bainite. The second type of bainite is characterized by precipitate free zone (marked in red in Fig. 13b). It is formed at the beginning of transformation stage by coalescence of bainite plates [23]. According to Bhadeshia et al. [24], the coalesced bainite is created by dissolution of filmlike austenite, which is present at bainite grain boundaries. This leads to a growth of bainite plate thickness by merging process of thin plates. The excess carbon from the dissolved austenite can precipitate in a central part of bainite as cementite or undergo partitioning to retained austenite. Because the coalesced bainite plates are very thick this partitioning may occur only at γ/α borders. That’s why the cementite precipitation inside bainite is more common. This is the reason for precipitate-free zones in coalesced bainite. Authors also observed that the coalesced bainite is present during isothermal heat treatments performed at temperatures lower than 400 °C [25]. The reason for this is a driving force necessary for the transformation in presence of strain energy associated with the bainite transformation. Larger plates exhibit the higher deformation energy, which need a sufficiently large driving force to growth. That’s why the coalesced bainite is not present at 400 °C. At this temperature, the driving force is too low for plates’ coalescence.
In case of 350 °C, the retained austenite is present both as thin layers and MA constituents. According to the XRD analysis (presented in Table 3), the amount of retained austenite is higher at 400 °C than at 350 °C. This is due to prior martensite formation before an isothermal step. The martensite transformation reduces the amount of austenite which may undergo bainite transformation. Normally without the martensite, a higher amount of retained austenite is expected at 350 °C. Moreover, one can see that a carbon enrichment of the austenite is higher at 350 °C, which is in accordance with theoretical calculations (the higher driving force). According to the obtained results, the carbon concentration in austenite is higher compared to the calculations.
For 4MnNb steels, the microstructure is composed of bainite, fresh and tempered martensite and retained austenite. Figure 14 presents the microstructure of 4MnNb steel at 400 °C. In this case, the microstructure is slightly different compared to 3MnNb steel. The main difference is the presence of fresh and tempered martensite. The fresh martensite is formed during cooling to room temperature, and at the same time, it undergoes tempering.
The tempered martensite comes from the fresh martensite formed at the beginning of transformation. This martensite is influenced by temperature for the longest time, and some tempering effect occurs during cooling to room temperature. Another difference is retained austenite morphology. In this treatment, the retained austenite is mainly present as blocks and MA constituents. Due to the martensite presence, it is hard to determine the exact amount of bainite formed during isothermal holding at different temperatures. That is why the amount of α phases (bainite and fresh martensite) is listed together in Table 4.
At 350 °C, the microstructure (Fig. 15) is similar to that obtained at 400 °C. According to the dilatometry, a smaller fraction of fresh martensite should be expected. In this temperature, a tempered martensite can be seen. Because the Ms temperature in this case was lower by 40 °C, the martensite undergoes tempering for shorter time. That’s why the visible effects are stronger at 400 °C. Similarly to 3MnNb 350 °C treatment, the coalesced bainite is also present. The retained austenite is located in MA constituents and as thin layers between bainite laths. Blocky retained austenite occurs too. According to the XRD analysis of the 4MnNb steel (Table 4), the retained austenite amount was higher in a sample isothermally treated at 350 °C. This is due to higher driving force of bainite transformation (hence a higher carbon enrichment of austenite). The measured carbon concentration in retained austenite proves this statement. An amount measured for 4MnNb is lower compared to 3MnNb steel. This is according to T0 curves showing that manganese shifts the curve to the left (to a lower carbon enrichment). At the same time, the amount of retained austenite at 400 °C (10%) is the lowest of all treatments. A lower temperature results in a higher amount of retained austenite (~ 13%), which is the same amount as for the 3MnNb steel at 400 °C.
Conclusions
A temperature and a manganese content have a high impact on the isothermal bainitic transformation kinetics in lean medium-Mn steels. At 350 °C, a higher amount of bainite is formed during the isothermal step due to the increased driving force of the transformation. The higher amount of bainite stimulates an increase in carbon content of the austenite increasing its thermal stability and corresponding volume fraction. The manganese content has an opposite effect. The increase of 0.5% Mn decreases the driving force of the bainitic transformation. The transformation kinetics is slower due to a retarding effect of Mn resulting in longer time necessary to finish bainitic transformation.
Martensite formed before an isothermal step also affects the bainite transformation kinetics. The martensite deforms the austenite increasing its dislocation density. The acceleration of bainitic transformation kinetics occurs due to the higher amount of places for bainite nucleation. This effect is present for 3MnNb steel treated at 350 °C. As the dilatometry showed the transformation kinetics was faster compared to the sample without martensite.
References
Speer J, Rana R, Matlock D, Glover A, Thomas G, De Moor E. Processing variants in medium-Mn steels. Metals. 2019. https://doi.org/10.3390/met9070771.
Lee YK, Han J. Current opinion in medium manganese steel. Mater Sci Technol. 2015. https://doi.org/10.1179/1743284714Y.0000000722.
Xiong ZP, Kostryzhev AG, Saleh AA, Chen L, Pereloma EV. Microstructures and mechanical properties of TRIP steel produced by strip casting simulated in the laboratory. Mater Sci Eng A. 2016. https://doi.org/10.1016/j.msea.2016.03.106.
Ma Y. Medium-manganese steels processed by austenite-reverted-transformation annealing for automotive applications. Mater Sci Technol. 2017. https://doi.org/10.1080/02670836.2017.1312208.
Grajcar A, Kilarski A, Kozłowska A. Microstructure-property relationship in thermomechanically processed medium-Mn steels with high Al content. Metals. 2018. https://doi.org/10.3390/met8110929.
Klueh RL, Maziasz PJ, Lee EH. Manganese as an austenite stabilizer in Fe–Cr–Mn–C steels. Mater Sci Eng A. 1988. https://doi.org/10.1016/0025-5416(88)90539-3.
Król M, Staszuk M, Mikuszewski T, Kuc D. Refinement effect of RE in light weight Mg–Li–Al alloys. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7290-z.
Santajuana MA, Eres-Castellanos A, Ruiz-Jimenez V, Allain S, Geandier G, Caballero FG, Garcia-Mateo C. Quantitative assessment of the time to end bainitic transformation. Metals. 2019. https://doi.org/10.3390/met9090925.
Farahani H, Xu W, Van der Zwaag S. Prediction and validation of the austenite phase fraction upon intercritical annealing of medium Mn steels. Metall Mater Trans A. 2015. https://doi.org/10.1007/s11661-015-3081-3.
Steineder K, Schneider R, Krizan D, Beal C, Sommitsch C. Comparative investigation of phase transformation behavior as a function of annealing temperature and cooling rate of two medium-Mn steels. Steel Res Int. 2015. https://doi.org/10.1002/srin.201400551.
Grajcar A, Zalecki W, Burian W, Kozłowska A. Phase equilibrium and austenite decomposition in advanced high-strength medium-Mn bainitic steels. Metals. 2016. https://doi.org/10.3390/met6100248.
Bhadeshia HKDH. A thermodynamic analysis of isothermal transformation diagrams. Met Sci. 2013. https://doi.org/10.1179/030634582790427217.
Grajcar A, Zalecki W, Skrzypczyk P, Kilarski A, Kowalski A, Kołodziej S. Dilatometric study of phase transformations in advanced high-strength bainitic steel. J Therm Anal Calorim. 2014. https://doi.org/10.1007/s10973-014-4054-2.
Guo H, Feng X, Zhao A, Li Q, Ma J. Influence of prior martensite on bainite transformation, microstructure, and mechanical properties in ultra-fine bainitic steel. Materials. 2019. https://doi.org/10.3390/ma12030527.
Navarro-Lopez A, Sietsma J, Santofimia MJ. Effect of prior athermal martensite on the isothermal transformation kinetic below Ms in a low-C high-Si steel. Metall Mater Trans A. 2016. https://doi.org/10.1007/s11661-015-3285-6.
Gong W, Tomota Y, Harjo S, Su YH, Aizawa K. Effect of prior martensite on bainite transformation in nanobainite steel. Acta Mater. 2015. https://doi.org/10.1016/j.actamat.2014.11.029.
Tian J, Xu G, Zhou M, Hu H, Wan X. The effects of Cr and Al addition on transformation and properties in low-carbon bainitic steels. Metals. 2017. https://doi.org/10.3390/met7020040.
Caballero FG, Bhadeshia HKDH. Very strong bainite. Curr Opin Solid State Mater Sci. 2004. https://doi.org/10.1016/j.cossms.2004.09.00515.
Guo H, Zhou P, Zhao A, Zhi C, Ding R, Wang J. Effects of Mn and Cr on microstructure and mechanical properties of low temperature bainitic steel. J Iron Steel Res Int. 2017. https://doi.org/10.1016/S1006-706X(17)30042-0.
Farahani H, Xu W, Zwaag S. Predicting the cooperative effect of Mn–Si and Mn–Mo on the incomplete bainite formation in quaternary Fe–C alloys. Philos Mag Lett. 2018. https://doi.org/10.1080/09500839.2018.1515505.
Grajcar A, Kamińska M, Opiela M, Skrzypczyk P, Grzegorczyk B, Kalinowska-Ozgowicz E. Segregation of alloying elements in thermomechanically rolled medium-Mn multiphase steel. J Achiev Mater Manuf Eng. 2012;55:256–64.
Xiaochuan X, Chen B, Wang H, Wang L. The effect of morphology on the stability of retained austenite in quenched and partitioned steel. Scr Mater. 2013. https://doi.org/10.1016/j.scriptamat.2012.11.003.
Pak JH, Bhadeshia HKDH, Larlsson L, Keehan E. Coalesced bainite by isothermal transformation of reheated weld metal. Sci Technol Weld Join. 2008. https://doi.org/10.1179/136217108X338926.
Bhadeshia HKDH, Keehan E, Karlsson L, Andren HO. Coalesced bainite. Trans Indian Inst Met. 2006;59:689–94.
Bhadeshia HKDH. Bainite in steels. 3rd ed. London: Institute of Materials; 1992.
Acknowledgements
This work is supported by own scholarship fund of Silesian University of Technology in 2019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morawiec, M., Ruiz-Jimenez, V., Garcia-Mateo, C. et al. Thermodynamic analysis and isothermal bainitic transformation kinetics in lean medium-Mn steels. J Therm Anal Calorim 142, 1709–1719 (2020). https://doi.org/10.1007/s10973-020-10259-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10259-z