Abstract
This work presents insights into the manganese influence on the driving force and bainite transformation kinetics. Three different medium-Mn steels were subjected to theoretical calculations and dilatometric study in order to determine the Mn impact on bainite formation. The theoretical approach shows that the increase of manganese leads to a lower bainite fraction formed during the isothermal stage. This implicates the carbon enrichment of the austenite during thermal treatment. The less bainite is formed, the higher is the fraction of residual austenite which enrichment of carbon is globally low. Meanwhile, the manganese influences the incubation and transformation time. As the manganese content increases, the incubation period and formation time of bainite are longer because the chemical driving force essential to start and complete austenite into bainite transformation decreases. This was proved by theoretical calculations and dilatometric analysis, which show that even a small increase in manganese content leads to a longer time necessary to occur the bainitic transformation. For the steel containing 5% manganese, the driving force was too small that the transformation could occur even after 3 h. Additionally, the XRD analysis was conducted to determine the retained austenite fraction and its carbon enrichment. These results were compared with the theoretical values to determine the accuracy of the applied model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The continuous development of steel for the automotive industry is conditioned by still rising environmental and safety requirements. At the same time, the cost of materials needs to be limited to prevent the drastic increase in the price of cars. For that reason, scientists around the world are trying to develop new grades of steel by changing its chemical composition and/or thermal/thermomechanical treatment [1,2,3,4,5,6]. Since the parts that constitute the car body are made of steel sheets a lot of different approaches to produce them were introduced. Currently, advanced high strength steels (AHSS) are extensively used in the automotive industry. These steels are characterized by multiphase microstructures, exhibiting high strength and good plasticity [7,8,9]. The AHSS steels are divided into three different generations. The first generation states dual-phase (ferrite and martensite), complex phase (ferrite, bainite, martensite, and retained austenite), and TRIP-assisted (Transformation-induced plasticity) steels [1,2,3]. The second-generation constitutes high manganese steels which have a structure composed of 100% austenite at room temperature. This is the effect of manganese content between 22 and 27% [4, 5]. The 2nd generation of AHSS steels exhibits enhanced mechanical properties; however, the main drawback is their high cost. It results in limited application of these steels in the car body. To achieve a compromise between price and mechanical properties, the third generation of AHSS steel is under development. These steels contain from 3 to 12% of manganese and depending on the treatment can exhibit the microstructure of ferrite and austenite, bainite and austenite or both of them, with a small fraction of martensite [10, 11]. This allows for a reduction of the steel price and obtaining a good combination between strength and plasticity. In the case of the 1st and 3rd generation of steels, the most important phase is retained austenite, which is responsible for the TRIP effect [1,2,3]. During deformation the retained austenite transforms into martensite, resulting in a local increase in strength, that delays and even prevents necking [4,5,6,7]. This effect takes place as long as retained austenite with certain degree of mechanical stability is present in the microstructure.
To obtain the microstructure composed of various phases with retained austenite in the 3rd generation of AHSS steels, there are a few approaches that can be used. The first one commonly used for cold-rolled steel sheets is intercritical annealing [11,12,13]. This treatment consists of heating the material to intercritical temperature range between Ac1 and Ac3, soaking at this temperature for some time, and final cooling to room temperature. At the intercritical temperature region, the microstructure is composed of ferrite and austenite, which further during annealing gradually transforms into austenite. A limited carbon solubility in ferrite leads to carbon diffusion into austenite increasing its thermal stability [13]. Concurrently, the manganese partitioning takes place which also increases austenite thermal stability, until the final fast cooling stage began. Another approach used into producing 3rd generation of AHSS steels is conducting a thermal process with heating above Ac3 temperature, full austenitization, held for some time, then applying a sufficiently fast cooling rate, avoiding ferrite/pearlite transformation to a temperature within the bainite formation regime, and applying an isothermal treatment [14, 15]. During bainitic transformation, there is carbon diffusion from the C supersaturated bainitic ferrite plates into the remaining austenite. Accordingly to this relation, it is possible to control a fraction of retained austenite and its thermal stability via changing the chemical composition of steel and the isothermal temperature. Since manganese is a strong austenite stabilizer, it also affects the bainite transformation kinetics. Therefore, a better understanding in how a level of manganese influences the chemical driving force of austenite into bainite transformation is crucial to further develop the 3rd generation of AHSS.
In this work, the analysis regarding the effect of different manganese content in medium-Mn steels on the chemical driving force and bainite transformation kinetics was carried out. Furthermore, the influence of retained austenite fraction and its thermal stability was also analysed. This knowledge is important for the determination of the best heat treatment for steels containing a higher amount of manganese.
Materials and experimental procedure
Three high-aluminium Nb-bearing steels with different manganese concentrations (3–5 mass%) were used in this investigation. The chemical compositions of the steel grades are given in Table 1.
The identification of the steels is made on the basis of the Mn content, i.e. 3MnNb, 4MnNb, and 5MnNb. Equivalent to the effect that Si addition above 1 mass% has on preventing carbide formation during bainitic transformation, a high aluminium content, ≈1.6 mass%, has been added with the same purpose. Such carbide inhibition in turn leads to higher enrichment of carbon in the remaining austenite, enhancing its stability. Additionally, it decreases the density of the steel, which results in a decrease in the mass of the car body elements. Molybdenum increases hardenability of the steel and the microaddition of Nb leads to grain refinement of the final microstructure via control of the prior austenite grain size by pinning effect exerted by the Nb precipitates. The theoretical changes in chemical driving force and bainite transformation kinetics, depending on a Mn content, were performed using the MUCG83 computer program [16, 17].
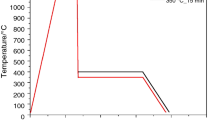
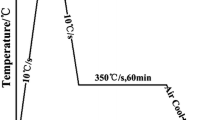
The applied heat treatment to obtain the bainitic microstructures for all the steels is presented in Fig. 1. The parameters were selected according to theoretical calculations carried out using JMatPro software and already presented in [7]. The samples were heated up to 1100 °C at a rate of 3°Cs−1 and hold at this temperature for 300 s to homogenise the austenite microstructure. After this step, the material was cooled at a rate of 60°Cs−1 to an isothermal holding temperature of 400 °C. This cooling was fast enough to ensure that no ferrite transformation could occur. The 400 °C temperature was selected, as according to calculations, the bainite formation is possible at this temperature for all analysed steels [7]. The duration of the isothermal step was fixed as 900 s, after which a 1°Cs−1 cooling rate down to room T was applied.
All heat treatments were performed using 4 mm diameter and 10 mm length cylindrical samples in a Bähr 805A high-resolution dilatometer. Cooling is applied by blowing helium to the sample, which was used as a quenching gas and the temperature was controlled by a type K thermocouple welded to the central part of the sample surface. From the obtained data, critical transformation temperatures, Ac1, Ac3, and Ms, and the kinetics of bainitic transformation were determined using the methodology presented in the work [18].
X-ray diffraction measurements were carried out with a Bruker AXS D8 diffractometer equipped with a Co X-ray tube. A current of 30 mA and a voltage of 40 kV were employed as tube settings. XRD data were collected over a 2θ range of 35–135° with a step size of 0.01°. Samples were metallographically prepared for XRD by using standard metallographic procedures, followed by etching in 3% Nital and polishing cycles. These cycles are useful to remove any stress/strain-induced martensite formed during the grinding step.
Results and discussion
Theoretical calculations
As a first approach, and to better understand and anticipate the influence of the manganese content on the bainite transformation, a set of theoretical calculations using MUCG83 software were performed. First, the analysis of the T0 line was carried out (see Fig. 2). This line represents the maximum C enrichment in austenite, above which the bainitic transformation is thermodynamically impossible to proceed further [19]. According to the calculations, increasing the manganese content shifts the T0 line to the left. That means the more manganese is in steel, the lower amount of bainite could be formed. To determine the maximum volume fraction of bainite, it is necessary to mark the bulk carbon concentration in the steel in Fig. 2. The distance between the bulk concentration and the equilibrium point from the T0 line for selected manganese content corresponds to the amount of bainite that can be formed. When the curves are shifted to the left (means that Mn is increased), the distance is shorter; therefore less bainite could be created during isothermal holding. The equilibrium point marked in Fig. 2 stands for austenite carbon concentration in which the bainitic transformation will stop. At this point, the free Gibbs energies of γ and α phases are equal; therefore, the chemical driving force is zero and the transformation could not proceed. This is a so-called incomplete reaction phenomenon (there is no possibility to obtain 100% of bainite in the final microstructure), which is widely described in other work [20].
According to the obtained results, it is possible to determine the maximum carbon enrichment of the austenite (Xγ) as a function of the manganese content in the steel. These values are presented in Table 2. If no other reaction interacts with the bainitic transformation, the carbon concentration of retained austenite can be determined as a function of the steel average carbon concentration and the bainitic ferrite volume fraction, using the following equation [21]:
where \(\overline{X }\) is the average carbon concentration of the alloy, s is the concentration of carbon in solid solution in the bainitic ferrite (s ≈ 0.03 mass%) [21]. The lever rule equation applied to the T0 line, that allows calculating the maximum volume fraction of bainite, is as follows:
In Fig. 3, the results of calculations from Table 2 are presented. Figure 3a shows the change of the maximum bainite fraction depending on the manganese content in the steel. One can observe that the higher manganese content in steel, the lower is maximum bainite fraction. A similar effect was reported by Guo et al. [22] for steels with elevated Mn and Cr contents. Knowing the bainite volume fraction after the heat treatment one can easily calculate the retained austenite volume fraction. Furthermore, based on the carbon and manganese concentration in austenite, it is possible to calculate the martensite start temperature (Ms) in accordance with the recently developed model by Kaar et al. [23] for advanced medium-Mn steels, as follows:
In Eq. (3), carbon and manganese are varying variables and the rest of the elements are assumed as constants for the three investigated steels. The results of Ms temperature calculations are presented in Fig. 3b. One can see that with increasing manganese content the austenite fraction also increases and that of bainite decreases. As the fraction of austenite increases, the Ms temperature also rises. This is the result of low carbon concentration which is widely redistributed in the remaining austenite fraction. One can conclude that the increase in manganese content leads to a higher volume fraction of austenite, which is poorly carbon-enriched, and therefore has lower thermal stability.
Another aspect of the manganese influence on the bainite transformation kinetics is the incubation time. A TTT diagram was calculated for all three steels (see Fig. 4), which demonstrates that the manganese decreases the start temperatures of bainitic (Bs) and martensitic (Ms) phase transformations. When the manganese content is increased from 3.1 to 5.0%, the Bs and Ms temperatures decrease by 72.2 °C and 70 °C, respectively. This is the effect of austenite stabilization by manganese [6]. For example, for the 3MnNb steel, the bainite needs 9 s of incubation time to start transformation. When the manganese content was increased, the incubation time also increased to 15 and 50 s. The same results were reported by Llopis [24], which determine that Mn retards bainite transformation like Ni and Cr. It means that the manganese decreases the driving force of phase transformations and therefore longer incubation times are needed.
Dilatometric analysis
To determine the kinetics of phase transformations for the investigated steels the dilatometric studies were carried out. The bainite formation and austenite stability analyses were performed on the basis of dilatometric curves from experiments with isothermal holding followed by cooling to room temperature. Figure 5 presents the results of dilatometric analysis during the isothermal stage. One can see from the kinetics of the bainitic transformation for the 3MnNb steel that there is no incubation period. This steel is characterized by the strongest dilatometric signal during the 900 s of isothermal treatment. It means that 3MnNb steel has the highest amount of bainite formed during the isothermal holding. After the 500 s the curve is flat, which implies that the bainitic transformation was finished. The 4MnNb steel exhibits some small incubation period before the start of the transformation ≈100 s. The slope of the dilatometric curve is smaller compared to the 3MnNb steel, and therefore, the transformation was slower in this case. Moreover, after 900 s the relative change in length (RCL) is still rising indicating that bainitic transformation has not reached its completion. On the other hand, for the 5MnNb steel, it is clear that bainitic transformation has not even started after 900 s of isothermal holding. This is visible in Fig. 5b, where the level of RCL is neglectable compared to the two other steels. Based on the presented results, one can see that manganese strongly slows down the kinetics of bainitic transformation. The increase of manganese content with 0.5%, results in the presence of incubation time and incomplete reaction phenomenon in the considered time during bainite formation. When the manganese was increased from 3.1 to 5.0%, there is no transformation occurrence during 15 min of the isothermal holding at the temperature of 400 °C. This effect is also documented in other works [20, 22].
The reason for such behaviour is the difference in chemical driving force for γ to α transformation. According to the calculations (Fig. 6), the increase in a manganese content leads to a drop in the driving force for the transformation. The driving force for 3MnNb steel is − 1142 J mol−1; when the manganese content is increased to 3.6%, the driving force is −1024 J mol−1. For the highest manganese content of 5% the driving force is − 781 J mol−1. This result means that the increase of 1.9% manganese between 3 and 5Mn steels changes the chemical driving force by 361 J mol−1. That’s why the transformation needs more time when the Mn content is increased, which explains the increased incubation time for the higher manganese steels. The same conclusion was made by Leach et al. [25]. According to the results, manganese influences the critical driving force for the diffusionless process. As manganese was increased from 1 to 6%, a level of driving force dropped drastically.
Analysing Fig. 7, which represents the dilatometric curve during cooling to room temperature, it is possible to determine the thermal stability of austenite. If no signal is detected, it means that the austenite is sufficiently enriched in C and not transform to martensite. Such phenomenon occurs for 3MnNb steel, where no RCL increase is visible. However, looking at 4MnNb and 5MnNb steels, the increase is visible. This means the formation of fresh martensite occurring during cooling to room temperature. Moreover, it is visible that the signal from 4MnNb is much smaller compared to 5MnNb. Such result means that during cooling more martensite was formed in 5MnNb steel. Another information is the Ms temperature of both steels. The Ms temperature of 330 °C detected during cooling to room temperature in case of 5MnNb is the same as for the bulk steel. This means that no stabilization of austenite occurred during isothermal holding. On the other hand, the Ms of 4MnNb detected by dilatometry is smaller compared to the martensitic start temperature of the steel. The difference between Ms temperature is 20 °C. This means that very small stabilization occurred during the isothermal treatment. This is correlated to the result presented in Fig. 5, where the 4MnNb steel did not obtain a maximum possible level of bainite.
Further experiments to confirm the previous observations were performed in order to check if the stabilization of austenite and formation of higher bainite amount was still possible, by drastically increasing the time of the isothermal holding for 4MnNb and 5MnNb steels to 1 h and 3 h, respectively. Dilatometric results obtained during the isothermal holding, see Fig. 8, clearly show that when the time is increased to 1 h for 4MnNb steel, the transformation was almost finished. On the other hand, even after 3 h the 5MnNb steel did not show any sign of bainitic transformation. The level of dilatometric signal (Fig. 8b) is at a level of measurement noise and can not be correlated with bainitic transformation. These results come to support the previous observation that manganese plays a crucial role in slowing bainitic transformation kinetics.
When looking at the cooling step to room temperature (Fig. 9) of both steels, it can be seen that Ms temperature for 5MnNb steel is exactly the same as that of the bulk steel (based on chemical composition). This proves that even after 3 h no bainite was formed in the microstructure. However, the Ms temperature for 4MnNb steel decreased and at the same time the signal from the formation of fresh martensite is very small. This is presented in Fig. 8, which means that during 1 h treatment more bainite was formed increasing the carbon enrichment of austenite (increasing its thermal stability). However, the Ms temperature did not change much compared to the 15 min treatment. At the same time, the smaller signal from the fresh martensite formation is detected. This means that the microstructure exhibits zones of low and high carbon austenites. The smaller signal, with insignificant change in Ms temperature, could mean the transformation of low carbon austenite. At 15 min the amount of this austenite was higher; that’s why the signal was much stronger. Prolonging time to 60 min results in the carbon redistribution leading to a lower fraction of low carbon austenite, decreasing the signal.
Figure 10 presents the microstructures of the steels after the heat treatment. As mentioned previously, the 3MnNb steel is composed of bainite laths with retained austenite. No martensite was detected in the microstructure. When looking at the microstructures of 4MnNb steels, it can be seen that during 15 min isothermal annealing the microstructure is composed of martensite and bainite due to the slower transformation kinetics. When the time was increased to 60 min, the structure is composed mainly of bainite with some fraction of martensite. This is in accordance with the dilatometric study, where the longer time of isothermal step resulted in a very small dilatometric signal from the fresh martensite during cooling. For 5MnNb steel, in both cases (15 and 180 min), the microstructure is fully martensitic. This means that no retained austenite could be stabilized in the microstructure of the steel as no carbon enrichment could occur.
Comparing the calculations and experimental results (Fig. 11), it can be seen that some discrepancies are present. First, taking into account the retained austenite fraction (Fig. 11a), it can be seen that in case of 3MnNb steel, the theoretical and experimental values are similar. The difference is 2% and is correlated with the carbon content in the austenite (Fig. 11b). The experimental carbon enrichment is 0.92% and the calculated one is 0.8%. This is because in case of 15% austenite, the volume in which the carbon can be dissolved is higher; hence, the global level of it is lower (as explained before). For 4MnNb steel the difference is present. The calculated value of retained austenite amount is 20%, but the experimental ones are 11% and 12% for 15 and 60 min isothermal holdings, respectively. The same concerns a carbon enrichment. The experimental values are much higher compared to the theoretical ones indicating the higher thermal stability of austenite. As presented in the cooling curves to room temperature, in case of 15 min isothermal holding, a high signal from fresh martensite was detected. It means that the thermal stability of retained austenite was not enough. At the same time, the presence of the low and high carbon zones influences the final results. When the time was increased to 60 min, the retained austenite amount did not change much. However, the signal from the fresh martensite was much smaller. It would mean that the amount of low carbon zones decreased during isothermal holding. Looking at the change of carbon enrichment in austenite, for shorter time a level of carbon in retained austenite is 1.13%. When the time was longer, the amount of retained austenite did not change; however, the fresh martensite signal (Fig. 9) was much smaller. This is related to the carbon concentration change. When the time was longer, a level of carbon enrichment was 0.94. This means that a higher fresh martensite signal should be detected during cooling. It was not a case. It could mean that more bainite was formed, which resulted in the better carbon distribution in austenite. That’s why the carbon level dropped from 1.13 to 0.94%, when the time was longer, because carbon had more time for the redistribution. This resulted in a decrease in low carbon zones, resulting in a lower signal from fresh martensite.
The same observation was reported by Timokhina et al. [26], in steel containing 2% of Mn. They reported that at the beginning of the bainite transformation, two kinds of austenite are present. One with a higher carbon content near the newly formed bainitic ferrite. The second one far from the transformed bainitic ferrite, with a lower carbon content. Moreover, they reported that the prolongation of isothermal holding time increases the homogeneity of the austenite. In 5MnNb steels, as no transformation occurred, it is impossible to compare the results. No transformation means no carbon enrichment and no retained austenite fraction. The high signal from fresh martensite and exactly the same Ms temperature of the transformation prove this behaviour.
Conclusions
Based on the presented results, it can be concluded that manganese has a crucial impact on the bainite transformation kinetics. According to the presented results, it can be stated that:
-
The increase in manganese content results in a lower bainite fraction. It leads to lower thermal stability of retained austenite,
-
Manganese influences also the incubation time for the bainite transformation. The incubation time is increased together with the increased manganese content,
-
The bainite transformation kinetics is strongly dependent on the manganese content. The higher is the level of manganese in steel, the longer time is necessary for bainite transformation to be finished. For the lowest Mn content of 3% the transformation finishes after around 500 s, where for the 5% Mn, it has not started even after 3 h. According to the calculations this is due to the drop of chemical driving force for the austenite to bainite transformation. Between 3 and 5MnNb the difference in a driving force was 361 J mol−1.
The higher amount of manganese leads to the higher amount of austenite during isothermal holding. This results in a lower carbon enrichment level in it, which decreases the thermal stability of austenite. According to the presented results of bainitic isothermal holding, the level of manganese should be limited to 3.6%. The higher manganese content results in very long times necessary for bainite transformation to occur, which is not acceptable from an industrial point of view.
References
Speer J, Rana R, Matlock D, Glover A, Thomas G, De Moor E. Processing variants in medium-Mn steels. Metals. 2019. https://doi.org/10.3390/met9070771.
Lee YK, Han J. Current opinion in medium manganese steel. Mater Sci Technol. 2015. https://doi.org/10.1179/1743284714Y.0000000722.
Xiong ZP, Kostryzhev AG, Saleh AA, Chen L, Pereloma EV. Microstructures and mechanical properties of TRIP steel produced by strip casting simulated in the laboratory. Mater Sci Eng A. 2016. https://doi.org/10.1016/j.msea.2016.03.106.
Jabłońska MB, Kowalczyk K. Microstructural aspects of energy absorption of high manganese steels. Proc Manufact. 2019. https://doi.org/10.1016/j.promfg.2018.12.049.
Bleck W, Haase C. Physical metallurgy of high manganese steels. Metals. 2019. https://doi.org/10.3390/met9101053.
Grajcar A, Kilarski A, Kozłowska A. Microstructure-property relationship in thermomechanically processed medium-Mn steels with high Al content. Metals. 2018. https://doi.org/10.3390/met8110929.
Grajcar A, Zalecki W, Skrzypczyk P, Kilarski A, Kowalski A, Kołodziej S. Dilatometric study of phase transformations in advanced high-strength bainitic steel. J Therm Anal Calorim. 2014. https://doi.org/10.1007/s10973-014-4054-2.
Klueh RL, Maziasz PJ, Lee EH. Manganese as an austenite stabilizer in Fe–Cr–Mn–C steels. Mater Sci Eng A. 1988. https://doi.org/10.1016/0025-5416(88)90539-3.
Santajuana MA, Eres-Castellanos A, Ruiz-Jimenez V, Allain S, Geandier G, Caballero FG, Garcia-Mateo C. Quantitative assessment of the time to end bainitic transformation. Metals. 2019. https://doi.org/10.3390/met9090925.
Steineder K, Schneider R, Krizan D, Beal C, Sommitsch C. Comparative investigation of phase transformation behavior as a function of annealing temperature and cooling rate of two medium-Mn steels. Steel Res Int. 2015. https://doi.org/10.1002/srin.201400551.
Grajcar A, Zalecki W, Burian W, Kozłowska A. Phase equilibrium and austenite decomposition in advanced high-strength medium-Mn bainitic steels. Metals. 2016. https://doi.org/10.3390/met6100248.
Ma Y. Medium-manganese steels processed by austenite-reverted-transformation annealing for automotive applications. Mater Sci Technol. 2017. https://doi.org/10.1080/02670836.2017.1312208.
Farahani H, Xu W, Van der Zwaag S. Prediction and validation of the austenite phase fraction upon intercritical annealing of medium Mn steels. Metall Mater Trans A. 2015. https://doi.org/10.1007/s11661-015-3081-3.
Hu X, Mueller JJ, Sun X, De Moor E, Speer JG, Matlock DK, Ren Y. The in situ observation of phase transformation during intercritical annealing of a medium manganese advanced high strength steel by high energy X-ray diffraction. Front Mater. 2021. https://doi.org/10.3389/fmats.2021.621784.
Tian J, Xu G, Zhou M, Hu H, Wan X. The effects of Cr and Al addition on transformation and properties in low-carbon bainitic steels. Metals. 2017. https://doi.org/10.3390/met7020040.
Shen YF, Qiu LN, Sun X, Zuo L, Liaw PK, Raabe D. Effects of retained austenite volume fraction, morphology, and carbon content on strength and ductility of nanostructured TRIP-assisted steels. Mater Sci Eng A. 2015. https://doi.org/10.1016/j.msea.2015.04.030.
Bhadeshia HKDH. A thermodynamic analysis of isothermal transformation diagrams. Metal Sci. 1982. https://doi.org/10.1179/030634582790427217.
U. of Cambridge, NPL: Materials Algorithms Project. www.msm.cam.ac.uk/map/mapmain.html.
Kawulokova M, Smetana B, Zla S, Kalup A, Mazancova E, Vanova P, Kawulok P, Dobrovska J, Rosypalova S. Study of equilibrium and nonequilibrium phase transformations temperatures of steel by thermal analysis method. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-016-5780-4.
Bhadeshia HKDH. Bainite in steels: theory and practice. 3rd ed. IOM3: Maney Publishing. 1992.
Farahani H, Xu W, Zwaag S. Predicting the cooperative effect of Mn–Si and Mn–Mo on the incomplete bainite formation in quaternary Fe–C alloys. Philos Mag Lett. 2018. https://doi.org/10.1080/09500839.2018.1515505.
Caballero FG, Santofimia MJ, Capdevila C, Garcia-Mateo C, Garcia de Andres C. Design of advanced bainitic steels by optimization of TTT diagrams and T0 curves. ISIJ Int. 2006. https://doi.org/10.2355/isijinternational.46.1479.
Guo H, Zhou P, Zhao A, Zhi C, Ding R, Wang J. Effects of Mn and Cr on microstructure and mechanical properties of low temperature bainitic steel. J Iron Steel Res Int. 2017. https://doi.org/10.1016/S1006-706X(17)30042-0.
Kaar S, Steineder K, Schneider R, Krizan D, Sommitsch C. New Ms-formula for exact microstructural prediction of modern 3rd generation AHSS chemistries. Scrip Mater. 2021. https://doi.org/10.1016/j.scriptamat.2021.113923.
Llopis AM. Isothermal transformation studies on the effect of alloying elements (Mo, Ni, Cr, Mn, Al, Si, Co) in steels on the kinetics of the austenite to bainite transformation. United States. U.S. Department of Energy. 1976. Thesis. Web: https://www.osti.gov/biblio/5348804
Leach L, Kolmskog P, Hoglund L, Hillert M, Borgenstam A. Critical driving forces for formation of bainite. Metall Mater Trans A. 2018. https://doi.org/10.1007/s11661-018-4819-5.
Timokhina IB, Liss KD, Raabe D, Rakha K, Beladi H, Xiong XY, Hodgson PD. J Appl Crystallogr. 2016. https://doi.org/10.1107/S1600576716000418.
Acknowledgements
The financial support of the National Science Center, Poland, is gratefully acknowledged, grant no. 2017/27/B/ST8/02864.
Author information
Authors and Affiliations
Contributions
MM and AG contributed to the study conception and design. Material preparation, data collection and analysis were performed by MM, CG-M, JO and JAJ. The first draft of the manuscript was written by MM. Initial review was carried out by AG, C-G-M, JO and JAJ. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morawiec, M., Opara, J., Garcia-Mateo, C. et al. Effect of Mn on the chemical driving force and bainite transformation kinetics in medium-manganese alloys. J Therm Anal Calorim 148, 1567–1576 (2023). https://doi.org/10.1007/s10973-022-11664-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11664-2