Abstract
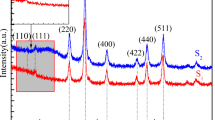
In this work, we report the synthesis of a new nanofluid (NF) based on magnetic nanoparticles (MNPS) synthesized by the coprecipitation method with high colloidal stability. The MNPS were functionalized with citric acid (Cac), and then, polyethylene glycol, 1000 (PEG1000), was bonded by polycondensation reactions with acid groups on the nanoparticles surface to increase the colloidal stability of the nanofluid. The MNPS were dispersed in an aqueous medium to obtain nanofluid-based magnetic nanoparticles in water (NF-MNPS-W) and in ethylene glycol to obtain nanofluid-based magnetic nanoparticles in ethylene glycol (NF-MNPS-E). The MNPS were characterized by X-ray diffraction and selected area electron diffraction, which confirmed the formation of the crystalline phase of Fe3O4. Transmission electron microscopy was used to confirm the size and morphology of the MNPS. The MNPS had an average diameter of 11.33 ± 3.68 nm. Infrared spectrum of the MNPS allowed the functionalization of the MNPS by Cac and then by PEG1000 to be proved. The colloidal stability of NF-MNPS-W (pH 8) and NF-MNPS-E was evaluated by measurement of Zeta potential (ζ) and dynamic light scattering (DLS) − 25 mV and 112 nm ± 1 nm, respectively. The DLS in the temperature function allowed the stability of the NF to be proved in working conditions.










Similar content being viewed by others
Abbreviations
- Cac:
-
Citric acid
- Dh:
-
Hydrodynamic diameter
- DLS:
-
Dynamic light scattering
- DTEM :
-
Diameter size
- FTIR:
-
Fourier transform infrared spectrum
- IEP:
-
Isoelectric point
- MNPS:
-
Magnetic nanoparticles
- MNPS-Cac:
-
Magnetic nanoparticles surface modified with citric acid
- MNPS-Cac-PEG1000:
-
Magnetic nanoparticles surface modified with citric acid and PEG1000
- NF:
-
Nanofluid
- NF-MNPS-E:
-
Nanofluid-based magnetic nanoparticles in ethylene glycol
- NF-MNPS-W:
-
Nanofluid-based magnetic nanoparticles in water
- NPS:
-
Nanoparticles
- PDI:
-
Polydispersity index
- PDIDLS :
-
Polydispersity index DLS
- PDITEM :
-
Polydispersity index TEM
- PEG1000:
-
Polyethylene glycol 1000
- SAED:
-
Selected area electron diffraction
- TEM:
-
Transmission electron microscopy
- XRD:
-
X-ray diffraction
- ζ:
-
Zeta potential
References
Hajatzadeh A, Aghakhani S, Afrand M, Mahmoudi B. An updated review on application of nanofluids in heat exchangers for saving energy. Energy Convers Manag. 2019;198:111886.
Mahbubul IM. Application of nanofluid 8.1. In: Preparation, characterization, properties, and application of nanofluid. 2019. https://doi.org/10.1016/b978-0-12-813245-6.00008-3.
Wahab A, Hassan A, Arslan M, Babar H, Usman M. Solar energy systems—potential of nanofluids. J Mol Liq. 2019;289:111049.
Kumar A, Subudhi S. Preparation, characterization and heat transfer analysis of nanofluids used for engine cooling. Appl Therm Eng. 2019;160:114092.
Al-rashed MH, Dzido G, Korpy M, Smo J, Wójcik J. Investigation on the CPU nanofluid cooling. Microelectron Reliab. 2016;63:159–65. https://doi.org/10.1016/j.microrel.2016.06.016.
Bahiraei M, Heshmatian S. Electronics cooling with nanofluids: a critical review. Energy Convers Manag. 2018;172:438–56.
Zhu K, Zhuo C, Yabo W, Hailong L, Xiaojing Z, Carsten F. Estimating the maximum energy-saving potential based on IT load and IT load shifting. Energy. 2017;138:902–9.
Wang Y, Wang B, Zhu K, Li H, He W, Liu S, Zhu K. Energy saving potential of using heat pipes for CPU cooling. Appl Therm Eng. 2018;143:630–8. https://doi.org/10.1016/j.applthermaleng.2018.07.132.
Sajid MU, Ali HM. Recent advances in application of nanofluids in heat transfer devices: a critical review. Renew Sustain Energy Rev. 2019;103:556–92.
Krishna VM, Kumar MS. ScienceDirect Numerical analysis of forced convective heat transfer of nanofluids in microchannel for cooling electronic equipment. Mater Today Proc. 2019;17:295–302.
Wong KV, De Leon O. Applications of nanofluids: current and future. Adv Mech Eng. 2010;2:519659.
Massart R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans Magn. 1981;17:1980–1.
Dolatabadi N, Rahmani R, Rahnejat H, Garner CP. Thermal conductivity and molecular heat transport of nanofluids. RSC Adv. 2019;9:2516–24. https://doi.org/10.1039/c8ra08987f.
Maji NC, Chakraborty J. Gram-scale green synthesis of copper nanowire powder for nanofluid applications. ACS Sustain Chem Eng. 2019;7:12376–88.
Zhu HT, Zhang CY, Tang YM, Wang JX. Novel synthesis and thermal conductivity of CuO nanofluid. J Phys Chem C. 2007;111:1646–50.
Saidur R, Leong KY, Mohammad HA. A review on applications and challenges of nanofluids. Renew Sustain Energy Rev. 2011;15:1646–68.
Farhana K, Rahman MM, Ramasamy D, Noor MM, Najafi G, Samykano M, Mahamude ASF. Improvement in the performance of solar collectors with nanofluids—a state-of-the-art review. Nano-Struct Nano-Objects. 2019;18:100276.
Sharshir SW, Mostafa ME, Essa FA, Kamal M, Ali A. Applications of nanofluids in solar energy: a review of recent advances. Renew Sustain Energy Rev. 2018;82:3483–502.
Azwadi N, Sidik C, Noor M, Mohd W, Mamat R. Recent advancement of nanofluids in engine cooling system. Renew Sustain Energy Rev. 2017;75:137–44.
Mahmoodi M, Kandelousi S. Cooling process of liquid propellant rocket by means of kerosene-alumina nanofluid. Propuls Power Res. 2016;5:279–86.
Kumar A, Kumar A, Rai A. Effects of Minimum Quantity Lubrication (MQL) in machining processes using conventional and nanofluid based cutting fluids: a comprehensive review. J Clean Prod. 2016;127:1–18.
Colangelo G, Favale E, Milanese M, De Risi A, Laforgia D. Cooling of electronic devices: nanofluids contribution. Appl Therm Eng. 2017;127:421–35.
Subudhi S, Kumar A. Application of nanofluids for radiator cooling. Encycl Renew Sustain Mater. 2019. https://doi.org/10.1016/b978-0-12-803581-8.11463-8.
Bozorg M, Fasano M, Cardellini A, Chiavazzo E, Asinari P. A review on the heat and mass transfer phenomena in nanofuid coolants with special focus on automotive applications. Renew Sustain Energy Rev. 2016;60:1615–33.
Wu JM, Zhao J. A review of nanofluid heat transfer and critical heat flux enhancement-Research gap to engineering application. Prog Nucl Energy. 2013;66:13–24.
Ghadimi A, Saidur R, Metselaar HSC. A review of nanofluid stability properties and characterization in stationary conditions. Int J Heat Mass Transf. 2011;54:4051–68.
Navarrete N, Gimeno-furió A, Forner-escrig J, Juliá JE, Mondragón R. Colloidal stability of molten salt -based nanofluids:dynamic light scattering tests at high temperature conditions. Powder Technol. 2019;352:1–10.
Fan Y, Chen Y, Liang X, Xu J, Deng T. Dispersion stability of thermal nanofluids. Prog Natural Sci Mater Int. 2017;27:531–42.
Briscoe WH. Current opinion in colloid, interface science depletion forces between particles immersed in nanofluids. Curr Opin Colloid Interface Sci. 2015;20:46–53.
Pilkington GA, Briscoe WH. Nanofluids mediating surface forces. Adv Colloid Interface Sci. 2012;182:68–84.
Sharma SK, Mital GS. Preparation and evaluation of stable nanofluids for heat transfer application: a review. Exp Therm Fluid Sci. 2016;76:202–12. https://doi.org/10.1016/j.expthermflusci.2016.06.029.
dos Santos CC, Viali WR, Viali EdaSN, Assis DR, Amantea BE. Aqueous nanofluids based on copper MPA: synthesis and characterization. Mater Res. 2017;20:104–10.
Abreu B, Lamas B. Experimental characterization of convective heat transfer with MWCNT based nanofluids under laminar flow conditions. Heat Mass Transf. 2014. https://doi.org/10.1007/s00231-013-1226-8.
Maskeen MM, Zeeshan A, Mehmood OU, Hassan M. Heat transfer enhancement in hydromagnetic alumina—copper/water hybrid nanofluid flow over a stretching cylinder. J Therm Anal Calorim. 2019;138(2):1127–36.
Akbari A, Hassan M. Experimental investigation of nanofluid stability on thermal performance and flow regimes in pulsating heat pipe. J Therm Anal Calorim. 2018;3:1835–47.
Azizi Z, Alamdari A, Doroodmand MM. Highly stable copper/carbon dot nanofluid. J Therm Anal Calorim. 2018;9:951–60.
Zareei M, Yoozbashizadeh H, Reza H, Hosseini M. Investigating the effects of pH, surfactant and ionic strength on the stability of alumina/water nanofluids using DLVO theory. J Therm Anal Calorim. 2018;1:1185–96.
Khairul MA, Doroodchi E, Azizian R, Moghtaderi B. Advanced applications of tunable ferrofluids in energy systems and energy harvesters: a critical review. Energ Convers Manage. 2017;149:660–74.
Felicia L, Vinod S, Philip J. Recent advances in magnetorheology of critical review. J Nanofluids. 2016;5(1):1–47. https://doi.org/10.1166/jon.2016.1203.
Hajiyan M, Ebadi S, Mahmud S, Biglarbegian M. Experimental investigation of the effect of an external magnetic field on the thermal conductivity and viscosity of Fe3O4—glycerol International Centre for Diffraction Data. J Therm Anal Calorim. 2018;1:1451–64.
Pin Y, Shameli K, Miyake M, Khairudin NBBtA, Mohamad SEBt, Naiki T, Lee KX. Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: a review. Arab J Chem. 2018. https://doi.org/10.1016/j.arabjc.2018.04.013.
Brandt JV, Piazza RD, dos Santos CC, Chacón JV, Amantéa BE, Pinto GC, Magnani M, Piva HL, Tedesco AC, Primo FL, Júnior MJ, Marques RFC. Synthesis and colloidal characterization of folic acid-modified PEG-b-PCL Micelles for methotrexate delivery. Colloids Surf B Biointerfaces. 2019;177:228–34.
Amantea BE, Piazza RD, Chacon JRV, dos Santos CC, Costa TP, Rocha CO, Brandt JV, Godoi DRM, Júnior MJ, Marques RFC. Esterification influence in thermosensitive behavior of copolymers PNIPAm- co-PAA and PNVCL-co-PAA in magnetic nanoparticles surface. Colloids Surf A. 2019;575:18–26.
De S, Mandal S. Physicochemical and engineering aspects surfactant-assisted shape control of copper nanostructures. Colloids Surf A. 2013;421:72–83.
Beriache M, Sidik NAC, Yazid MNAWN, Mamat R, Najafi G, Kefayati GHR. A review on why researchers apply external magnetic field on nanofluids. Int Commun Heat Mass Transf. 2016;78:60–7.
Lu A, Salabas EL, Schüth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angewandte. 2007. https://doi.org/10.1002/anie.200602866.
Okubo T, Particles E. Fundamentals of Colloid and Surface Chemistry. Colloidal Organization. 2015. https://doi.org/10.1016/b978-0-12-802163-7.00002-7.
Bajpai P. Biermann’s handbook of pulp and paper: volume 2: paper and board making. Colloid Surf Chem. 2018;19(1):381–400. https://doi.org/10.1016/b978-0-12-814238-7.00019-2.
Ohshima H. CHAPTER 1—Interaction of colloidal particles. In: Colloid and interface science in pharmaceutical research and development. Elsevier B.V.; 2014. https://doi.org/10.1016/b978-0-444-62614-1.00001-6.
Viali WR, Nunes ES, dos Santos CC, Fermin SWS, Aragón H, Coaquira JAH, Morais PC, Júnior MJ. PEGylation of SPIONs by polycondensation reactions: a new strategy to improve colloidal stability in biological media. J Nanoparticle Res. 2013. https://doi.org/10.1007/s11051-013-1824-x.
Abdelbar MF, Fayed TA, Meaz TM, Ebeid E-ZM. Molecular and biomolecular spectroscopy photo-induced interaction of thioglycolic acid (TGA)-capped CdTe quantum dots with cyanine dyes. SAA. 2016;168:1–11.
Clayton KN, Salameh JW, Wereley ST, Kinzer-Ursem TL. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics. 2016;10:054107.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds, part B, applications in coordination, organometallic, and bioinorganic chemistry. 2009.
Ferrer EG, Bichara LC, Gramajo B, Brand SA. Vibrational study and force field of the citric acid dimer based on the SQM methodology. Adv Phys Chem. 2011;11:347072-10.
Cuadro PD, et al. Reactive, functional polymers cross-linking of cellulose and poly (ethylene glycol) with citric acid. React Funct Polym. 2015;90:21–4.
Castillo PM, Mata MDe, Casula MF, Sánchez-alcázar JA, Zaderenko AP. PEGylated versus non-PEGylated magnetic nanoparticles as Camptothecin delivery system. Beilstein J Nanotechnol. 2014;5:1312–9. https://doi.org/10.3762/bjnano.5.144.
Tapadiya A, Vasanthan N. Crystallization and alkaline hydrolysis of poly (3-hydroxybutyrate) films probed by thermal analysis and infrared spectroscopy. Int J Biol Macromol. 2017;102:1130–7.
Alemdar A, Gungor N, Ece OI, Atici O. The rheological properties and characterization of bentonite dispersions in the presence of non-ionic polymer PEG. J Mater Sci. 2005;40:171–7.
Hunter RJ. Foundations of colloid science. 2nd ed. Oxford University Press; 2001.
Viali WR, Alcantara GB, Sartoratto PPC, Soler MAG, Mosiniewicz-Szablewska E, Andrzejewski B, Morais PC. Investigation of the molecular surface coating on the stability of insulating magnetic oils. J Phys Chem C. 2010;114(1):179–88.
Singh AK. Structure, synthesis, and application of nanoparticles. Eng Nanoparticles. 2016. https://doi.org/10.1016/B978-0-12-801406-6.00002-9.
Sun Z, Su F, Forsling W, Samskog P. Surface characteristics of magnetite in aqueous suspension. J Colloid Interface Sci. 1998;159:151–9.
Aguilar K, Garvín A, Lara-sagahón AV, Ibarz A. Ascorbic acid degradation in aqueous solution during UV–Vis irradiation. Food Chem. 2019;297:124864.
dos Santos CC, Viali WR, Viali ESN, Júnior MJ. Aqueous nanofluids based on thioglycolic acid-coated copper sulfide nanoparticles for heat-exchange applications. J Mol Liq. 2020;313:0167-7322. https://doi.org/10.1016/j.molliq.2020.113391.
Hunter RJ. Applications of the Zeta Potential-chapter 6. 1981. https://doi.org/10.1016/b978-0-12-361961-7.50010-9.
Shaw DJ. Introduction to colloid and surface chemistry. 1992. https://doi.org/10.1016/C2009-0-24070-0..
Funding
Funding was provided by FAPESP (Grant No. project 2015/126385).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
dos Santos, C.C., Viali, W.R., Viali, E.S.N. et al. Colloidal stability study of Fe3O4-based nanofluids in water and ethylene glycol. J Therm Anal Calorim 146, 509–520 (2021). https://doi.org/10.1007/s10973-020-10062-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10062-w