Abstract
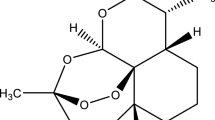
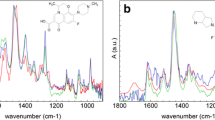
A series of five guest–host inclusion complexes containing the antimalarial and anticancer agent artesunate (ATS) were obtained and characterized in the present study. Different cyclodextrins (CDNs) were used as hosts [α-cyclodextrin (CDN1), β-cyclodextrin (CDN2), γ-cyclodextrin (CDN3), (2-hydroxypropyl)-β-cyclodextrin (CDN4) and (2-hydroxypropyl)-γ-cyclodextrin (CDN5)], and the formation of the adducts was simulated using molecular modelling. The results indicating the hypothetical formation of all complexes were confirmed on the prepared samples by FTIR spectroscopy and thermal analysis (TG—thermogravimetric/DTG—derivative thermogravimetric/HF—heat flow). Our results showed that the partially entrapment of ATS inside the cavity of each cyclodextrin is a consequence of H-bonds formation, electrostatic interactions (dipole–dipole) and hydration water substitution. Also, all complexes formed in a 1:1 molecular ratio presented with higher thermal stability than pure ATS, making the analysed adducts possible alternatives in the drug design process of new and improved pharmaceutical formulations containing ATS.





Similar content being viewed by others
References
Xie DY. Artemisia annua, artemisinin, and the Nobel Prize: beauty of natural products and educational significance. Sci Bull. 2016;61(1):42–4.
WHO. Treatment. In: Compendium of WHO malaria guidance-prevention, diagnosis, treatment, surveillance and elimination. World Health Organization. 2019. https://apps.who.int/iris/bitstream/handle/10665/312082/WHO-CDS-GMP-2019.03-eng.pdf?ua=1. Accessed 21 Dec 2019.
Efferth T. Artemisinin-second career as anticancer drug? World J Tradit Chin Med. 2015;1(4):2–25.
Jeong DE, Song HJ, Lim S, et al. Repurposing the anti-malarial drug artesunate as a novel therapeutic agent for metastatic renal cell carcinoma due to its attenuation of tumor growth, metastasis, and angiogenesis. Oncotarget. 2015;6(32):33046–64.
Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol. 2017;46:65–83.
Antoszczak M, Markowska A, Markowska J, Huczyński A. Old wine in new bottles: drug repurposing in oncology. Eur J Pharmacol. 2020. https://doi.org/10.1016/j.ejphar.2019.172784.
Wikman-Jorgensen PE, Henríquez-Camacho CA, Serrano-Villar S, Pérez-Molina JA. The role of artesunate for the treatment of urinary schistosomiasis in schoolchildren: a systematic review and meta-analysis. Pathog Glob Health. 2012;106(7):397–404.
Egbe-Nwiyi TN, Igwenagu E, Ndueidem UIT, Zira LJ. The therapeutic efficacy of artesunate and diminazene in the treatment of experimental Trypanosoma bruceibrucei infection in rats. Afr J Biomed Res. 2014;17(1):9–14.
Ho WE, Peh HY, Chan TK, Wong WSF. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther. 2014;142(1):126–39.
Ansari MT, Pervez H, Shehzad MT, Saeed-ul-Hassan S, Mehmood Z, Shah SNH, Razi MT, Murtaza G. Improved physicochemical characteristics of artemisinin using succinic acid. Acta Pol Pharm. 2014;71(3):451–62.
Scheibel LW. WHO model prescribing information, drugs used in parasitic diseases. J Parasitol. 1991;77(6):957.
Kouakou YI, Tod M, Leboucher G, Lavoignat A, Bonnot G, Bienvenu AL, Picot S. Systematic review of artesunate pharmacokinetics: implication for treatment of resistant malaria. Int J Infect Dis. 2019;89:30–44.
Morris CA, Duparc S, Borghini-Fuhrer I, Jung D, Shin CS, Fleckenstein L. Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar J. 2011;10(1):263.
von Hagens C, Walter-Sack I, Goeckenjan M, et al. Prospective open uncontrolled phase I study to define a well-tolerated dose of oral artesunate as add-on therapy in patients with metastatic breast cancer (ARTIC M33/2). Breast Cancer Res Treat. 2017;164(2):359–69.
König M, Von Hagens C, Hoth S, Baumann I, Walter-Sack I, Edler L, Sertel S. Investigation of ototoxicity of artesunate as add-on therapy in patients with metastatic or locally advanced breast cancer: new audiological results from a prospective, open, uncontrolled, monocentric phase I study. Cancer Chemother Pharmacol. 2016;77(2):413–27.
Ericsson T, Blank A, Von Hagens C, Ashton M, Äbelö A. Population pharmacokinetics of artesunate and dihydroartemisinin during long-term oral administration of artesunate to patients with metastatic breast cancer. Eur J Clin Pharmacol. 2014;70(12):1453–63.
Deeken JF, Wang H, Hartley M, et al. A phase I study of intravenous artesunate in patients with advanced solid tumor malignancies. Cancer Chemother Pharmacol. 2018;81(3):587–96.
Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3(12):1023–35.
Duchêne D, Bochot A. Thirty years with cyclodextrins. Int J Pharm. 2016;514(1):58–72.
Vyas A, Saraf S, Saraf S. Cyclodextrin based novel drug delivery systems. J Incl Phenom Macrocycl Chem. 2008;62(1–2):23–422.
Gidwani B, Vyas A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Biomed Res Int. 2015;2015:1–15.
Circioban D, Ledeti A, Vlase G, Coricovac D, Moaca A, Farcas C, Vlase T, Ledeti I, Dehelean C. Guest–host interactions and complex formation for artemisinin with cyclodextrins: instrumental analysis and evaluation of biological activity. J Therm Anal Calorim. 2018;134(2):1375–84.
Şuta LM, Vlaia L, Vlaia V, Olariu I, Hǎdǎrugǎ DI, Mircioiu C. Study of the complexation behavior of tenoxicam with cyclodextrins. Farmacia. 2012;60(4):475–83.
Suta L-M, Vlaia L, Fulias A, Ledeti I, Hadaruga D, Mircioiu C. Evaluation study of the inclusion complexes of some oxicams with 2-hydroxypropyl-beta-cyclodextrin. Rev Chim. 2013;64(11):1279–83.
Loftsson T, Duchêne D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329(1–2):1–11.
Chadha R, Gupta S, Shukla G, Jain DVS, Pissurlenkar RRS, Coutinho EC. Interaction of artesunate with β-cyclodextrin: characterization, thermodynamic parameters, molecular modeling, effect of PEG on complexation and antimalarial activity. Results Pharma Sci. 2011;1(1):38–48.
Xie H, Yang B, Wang F, Zhao Y. Host–guest inclusion system of artesunate with β-cyclodextrin and its derivatives: characterization and antitumor activity. J Mol Struct. 2015;1085:90–6.
Al Hayder M, Sunderland VB. Effect of hydroxypropyl-ß-cyclodextrin complexation on the aqueous solubility and stability of artesunate. Int J Pharm Pharm Sci. 2014;6(7):598–601.
Zhang D, Zhou C, Lv P, Zhao Y, Liang J, Liao X, Yang B. Preparation and characterization of a novel host–guest complex based on folate-modified β-cyclodextrin and artesunate. Mater Sci Eng C. 2018;86:48–55.
Sbârcea L, Udrescu L, Ledeţi I, Szabadai Z, Fuliaş A, Sbârcea C. β-Cyclodextrin inclusion complexes of lisinopril and zofenopril: physicochemical characterization and compatibility study of lisinopril-β-cyclodextrin with lactose. J Therm Anal Calorim. 2016;123(3):2377–90.
Frisch MJ, Trucks GW, Schlegel HB et al. Gaussian 09. Wallingford CT: Gaussian, Inc. 2009.
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30(16):2785–91.
Schrodinger LLC. The AxPyMOL molecular graphics plugin for Microsoft PowerPoint, version 1.8, November 2015.
Schrodinger LLC. The JyMOL molecular graphics development component, version 1.8, November 2015.
Schrodinger LLC. The PyMOL molecular graphics system, version 1.8, November 2015.
Dassault Systèmes BIOVIA. BIOVIA workbook, release 2017; BIOVIA pipeline pilot, release 2017. San Diego: Dassault Systèmes; 2019.
Huey R, Morris GM, Olson AJ, Goodsell DS. A semiempirical free energy force field with charge-based desolvation. J Comput Chem. 2007;28(6):1145–52.
Circioban D, Ledeţi I, Vlase G, Ledeţi A, Axente C, Vlase T, Dehelean C. Kinetics of heterogeneous-induced degradation for artesunate and artemether. J Therm Anal Calorim. 2018;134(1):749–56.
Rachmawati H, Edityaningrum CA, Mauludin R. Molecular inclusion complex of curcumin–β-cyclodextrin nanoparticle to enhance curcumin skin permeability from hydrophilic matrix gel. AAPS PharmSciTech. 2013;14(4):1303–12.
Misiuk W, Tykocka A. Sensitive extractive spectrophotometric methods for the determination of nortriptyline hydrochloride in pharmaceutical formulations. Chem Pharm Bull (Tokyo). 2007;55(12):1655–61.
Aderibigbe B. Design of drug delivery systems containing artemisinin and its derivatives. Molecules. 2017;22(2):323.
Brusnikina M, Silyukov O, Chislov M, Volkova T, Proshin A, Mazur A, Tolstoy P, Terekhova I. Effect of cyclodextrin complexation on solubility of novel anti-Alzheimer 1,2,4-thiadiazole derivative. J Therm Anal Calorim. 2017;130(1):443–50.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Circioban, D., Ledeti, I., Suta, LM. et al. Instrumental analysis and molecular modelling of inclusion complexes containing artesunate. J Therm Anal Calorim 142, 1951–1961 (2020). https://doi.org/10.1007/s10973-020-09975-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09975-3