Abstract
Purpose
The purpose of this study were firstly to characterize the population pharmacokinetics of artesunate (ARS) and its active metabolite dihydroartemisinin (DHA) in patients with metastatic breast cancer during long-term (>3 weeks) daily oral ARS administration and secondly to study the relationship between salivary and plasma concentrations of DHA.
Methods
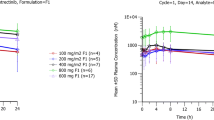
Drug concentration-time data from 23 patients, receiving oral ARS (100, 150, or 200 mg OD), was analyzed using nonlinear mixed effects modeling. A combined drug-metabolite population pharmacokinetic model was developed to describe the plasma pharmacokinetics of ARS and DHA in plasma. Saliva drug concentrations were incorporated as being directly proportional to plasma concentrations.
Results
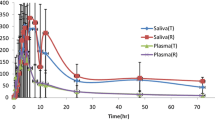
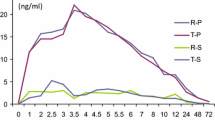
A first-order absorption model for ARS linked to a combined two-compartment disposition model for ARS and one-compartment disposition model for DHA provided the best fit to the data. No covariates were identified that could explain between-subject variability. A time-dependent increase in apparent elimination clearance of DHA was observed. Salivary DHA concentrations were proportionally correlated with total DHA plasma concentrations, with an estimated slope factor of 0.116.
Conclusions
Population pharmacokinetics of ARS and DHA in patients with breast cancer was well described by a combined drug-metabolite model without any covariates and with an increase in apparent elimination clearance of DHA over time. The estimated DHA saliva/plasma ratio was in good agreement with the reported DHA unbound fraction in human plasma. Saliva ARS concentrations correlated poorly with plasma concentrations. This suggests the use of saliva sampling for therapeutic drug monitoring of DHA. However, further studies are warranted to investigate the robustness of this approach.





Similar content being viewed by others
References
Navaratnam V, Mansor SM, Sit NW, Grace J, Li Q, Olliaro P (2000) Pharmacokinetics of artemisinin-type compounds. Clin Pharmacokinet 39(4):255–270
(2010) Guidelines for the treatment of malaria, 2nd edn. World Health Organization (WHO)
Benakis A, Paris M, Loutan L, Plessas CT, Plessas ST (1997) Pharmacokinetics of artemisinin and artesunate after oral administration in healthy volunteers. Am J Trop Med Hyg 56(1):17–23
Price RN, Nosten F, Luxemburger C, van Vugt M, Phaipun L, Chongsuphajaisiddhi T, White NJ (1997) Artesunate/mefloquine treatment of multi-drug resistant falciparum malaria. Trans R Soc Trop Med Hyg 91(5):574–577
Price R, van Vugt M, Phaipun L, Luxemburger C, Simpson J, McGready R, ter Kuile F, Kham A, Chongsuphajaisiddhi T, White NJ, Nosten F (1999) Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives. Am J Trop Med Hyg 60(4):547–555
Ratcliff A, Siswantoro H, Kenangalem E, Maristela R, Wuwung RM, Laihad F, Ebsworth EP, Anstey NM, Tjitra E, Price RN (2007) Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet 369(9563):757–765
Efferth T (2007) Willmar Schwabe Award 2006: antiplasmodial and antitumor activity of artemisinin—from bench to bedside. Planta Med 73(4):299–309
Firestone GL, Sundar SN (2009) Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev Mol Med 11:e32
Hamacher-Brady A, Stein HA, Turschner S, Toegel I, Mora R, Jennewein N, Efferth T, Eils R, Brady NR (2011) Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J Biol Chem 286(8):6587–6601
Singh NP, Lai H (2001) Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci 70(1):49–56
Lai H, Nakase I, Lacoste E, Singh NP, Sasaki T (2009) Artemisinin-transferrin conjugate retards growth of breast tumors in the rat. Anticancer Res 29(10):3807–3810
Singh NP, Lai HC, Park JS, Gerhardt TE, Kim BJ, Wang S, Sasaki T (2011) Effects of artemisinin dimers on rat breast cancer cells in vitro and in vivo. Anticancer Res 31(12):4111–4114
Mao H, Gu H, Qu X, Sun J, Song B, Gao W, Liu J, Shao Q (2013) Involvement of the mitochondrial pathway and Bim/Bcl-2 balance in dihydroartemisinin-induced apoptosis in human breast cancer in vitro. Int J Mol Med 31(1):213–218
GLOBOCAN (2012) Estimated cancer incidence, mortality and prevalence worldwide in 2012. International Agency for Research on Cancer; World Health Organization (WHO)
Morris CA, Duparc S, Borghini-Fuhrer I, Jung D, Shin CS, Fleckenstein L (2011) Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar J 10:263
Ilett KF, Ethell BT, Maggs JL, Davis TM, Batty KT, Burchell B, Binh TQ, le Thu TA, Hung NC, Pirmohamed M, Park BK, Edwards G (2002) Glucuronidation of dihydroartemisinin in vivo and by human liver microsomes and expressed UDP-glucuronosyltransferases. Drug Metab Dispos 30(9):1005–1012
Na-Bangchang K, Krudsood S, Silachamroon U, Molunto P, Tasanor O, Chalermrut K, Tangpukdee N, Matangkasombut O, Kano S, Looareesuwan S (2004) The pharmacokinetics of oral dihydroartemisinin and artesunate in healthy Thai volunteers. Southeast Asian J Trop Med Public Health 35(3):575–582
Newton P, Suputtamongkol Y, Teja-Isavadharm P, Pukrittayakamee S, Navaratnam V, Bates I, White N (2000) Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob Agents Chemother 44(4):972–977
Teja-Isavadharm P, Watt G, Eamsila C, Jongsakul K, Li Q, Keeratithakul G, Sirisopana N, Luesutthiviboon L, Brewer TG, Kyle DE (2001) Comparative pharmacokinetics and effect kinetics of orally administered artesunate in healthy volunteers and patients with uncomplicated falciparum malaria. Am J Trop Med Hyg 65(6):717–721
Hassan Alin M, Ashton M, Kihamia CM, Mtey GJ, Bjorkman A (1996) Multiple dose pharmacokinetics of oral artemisinin and comparison of its efficacy with that of oral artesunate in falciparum malaria patients. Trans R Soc Trop Med Hyg 90(1):61–65
Ashton M, Hai TN, Sy ND, Huong DX, Van Huong N, Nieu NT, Cong LD (1998) Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab Dispos 26(1):25–27
Ashton M, Nguyen DS, Nguyen VH, Gordi T, Trinh NH, Dinh XH, Nguyen TN, Le DC (1998) Artemisinin kinetics and dynamics during oral and rectal treatment of uncomplicated malaria. Clin Pharmacol Ther 63(4):482–493
Khanh NX, de Vries PJ, Ha LD, van Boxtel CJ, Koopmans R, Kager PA (1999) Declining concentrations of dihydroartemisinin in plasma during 5-day oral treatment with artesunate for Falciparum malaria. Antimicrob Agents Chemother 43(3):690–692
van Agtmael MA, Cheng-Qi S, Qing JX, Mull R, van Boxtel CJ (1999) Multiple dose pharmacokinetics of artemether in Chinese patients with uncomplicated falciparum malaria. Int J Antimicrob Agents 12(2):151–158
Simonsson US, Jansson B, Hai TN, Huong DX, Tybring G, Ashton M (2003) Artemisinin autoinduction is caused by involvement of cytochrome P450 2B6 but not 2C9. Clin Pharmacol Ther 74(1):32–43
Svensson US, Ashton M, Trinh NH, Bertilsson L, Dinh XH, Nguyen VH, Nguyen TN, Nguyen DS, Lykkesfeldt J, Le DC (1998) Artemisinin induces omeprazole metabolism in human beings. Clin Pharmacol Ther 64(2):160–167
Asimus S, Hai TN, Van Huong N, Ashton M (2008) Artemisinin and CYP2A6 activity in healthy subjects. Eur J Clin Pharmacol 64(3):283–292
Elsherbiny DA, Asimus SA, Karlsson MO, Ashton M, Simonsson US (2008) A model based assessment of the CYP2B6 and CYP2C19 inductive properties by artemisinin antimalarials: implications for combination regimens. J Pharmacokinet Pharmacodyn 35(2):203–217
Birgersson S, Ericsson T, Blank A, von Hagens C, Ashton M (2014) A high-throughput liquid chromatographic-tandem mass spectrometric assay for quantification of artesunate and its metabolite dihydroartemisinin in human plasma and saliva. Bioanalysis. doi:10.4155/BIO.14.116
Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ. NONMEM user’s guides (1989–2009), ICON Development Solutions, Ellicott City, MD, USA
Lindbom L, Ribbing J, Jonsson EN (2004) Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Prog Biomed 75(2):85–94
Lindbom L, Pihlgren P, Jonsson EN (2005) PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Prog Biomed 79(3):241–257
Jonsson EN, Karlsson MO (1999) Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Prog Biomed 58(1):51–64
Beal SL, Sheiner LB (1982) Estimating population kinetics. Crit Rev Biomed Eng 8(3):195–222
Beal SL (2001) Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28(5):481–504
Ahn JE, Karlsson MO, Dunne A, Ludden TM (2008) Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn 35(4):401–421
Bergstrand M, Karlsson MO (2009) Handling data below the limit of quantification in mixed effect models. AAPS J 11(2):371–380
Lee IS, Hufford CD (1990) Metabolism of antimalarial sesquiterpene lactones. Pharmacol Ther 48(3):345–355
Batty KT, Le AT, Ilett KF, Nguyen PT, Powell SM, Nguyen CH, Truong XM, Vuong VC, Huynh VT, Tran QB, Nguyen VM, Davis TM (1998) A pharmacokinetic and pharmacodynamic study of artesunate for vivax malaria. Am J Trop Med Hyg 59(5):823–827
Gordi T, Xie R, Huong NV, Huong DX, Karlsson MO, Ashton M (2005) A semiphysiological pharmacokinetic model for artemisinin in healthy subjects incorporating autoinduction of metabolism and saturable first-pass hepatic extraction. Br J Clin Pharmacol 59(2):189–198
Shepard TA, Reuning RH, Aarons LJ (1985) Estimation of area under the curve for drugs subject to enterohepatic cycling. J Pharmacokinet Biopharm 13(6):589–608
Pétricoul O, Cosson V, Fuseau E, Marchand M (2007) Population models for drug absorption and enterohepatic recycling. In: Ette EI, Williams PJ (eds) Pharmacometrics: the science of quantitative pharmacology. John Wiley & Sons, Hoboken, pp 345–382
Tan B, Naik H, Jang IJ, Yu KS, Kirsch LE, Shin CS, Craft JC, Fleckenstein L (2009) Population pharmacokinetics of artesunate and dihydroartemisinin following single- and multiple-dosing of oral artesunate in healthy subjects. Malar J 8:304
Morris CA, Onyamboko MA, Capparelli E, Koch MA, Atibu J, Lokomba V, Douoguih M, Hemingway-Foday J, Wesche D, Ryder RW, Bose C, Wright L, Tshefu AK, Meshnick S, Fleckenstein L (2011) Population pharmacokinetics of artesunate and dihydroartemisinin in pregnant and non-pregnant women with malaria. Malar J 10:114
Davies NM, Takemoto JK, Brocks DR, Yanez JA (2010) Multiple peaking phenomena in pharmacokinetic disposition. Clin Pharmacokinet 49(6):351–377
Keiser J, Gruyer MS, Perrottet N, Zanolari B, Mercier T, Decosterd L (2009) Pharmacokinetic parameters of artesunate and dihydroartemisinin in rats infected with Fasciola hepatica. J Antimicrob Chemother 63(3):543–549
Li Q, Xie L, Zhang J, Weina PJ (2008) The distribution pattern of intravenous [(14)C] artesunate in rat tissues by quantitative whole-body autoradiography and tissue dissection techniques. J Pharm Biomed Anal 48(3):876–884
Le Thi DT, Le NH, Nguyen CH, Phan Thi D, Na-Bangchang K (2008) Pharmacokinetics of a five-day oral dihydroartemisinin monotherapy regimen in patients with uncomplicated falciparum malaria. Drug Metab Pharmacokinet 23(3):158–164
Diem Thuy LT, Ngoc Hung L, Danh PT, Na-Bangchang K (2008) Absence of time-dependent artesunate pharmacokinetics in healthy subjects during 5-day oral administration. Eur J Clin Pharmacol 64(10):993–998
Leskovac V, Theoharides AD (1991) Hepatic metabolism of artemisinin drugs—I. Drug metabolism in rat liver microsomes. Comp Biochem Physiol C 99(3):383–390
Liu T, Du F, Wan Y, Zhu F, Xing J (2011) Rapid identification of phase I and II metabolites of artemisinin antimalarials using LTQ-Orbitrap hybrid mass spectrometer in combination with online hydrogen/deuterium exchange technique. J Mass Spectrom 46(8):725–733
Asimus S, Elsherbiny D, Hai TN, Jansson B, Huong NV, Petzold MG, Simonsson US, Ashton M (2007) Artemisinin antimalarials moderately affect cytochrome P450 enzyme activity in healthy subjects. Fundam Clin Pharmacol 21(3):307–316
von Bahr C, Steiner E, Koike Y, Gabrielsson J (1998) Time course of enzyme induction in humans: effect of pentobarbital on nortriptyline metabolism. Clin Pharmacol Ther 64(1):18–26
Aps JK, Martens LC (2005) Review: the physiology of saliva and transfer of drugs into saliva. Forensic Sci Int 150(2–3):119–131
Gordi T, Hai TN, Hoai NM, Thyberg M, Ashton M (2000) Use of saliva and capillary blood samples as substitutes for venous blood sampling in pharmacokinetic investigations of artemisinin. Eur J Clin Pharmacol 56(8):561–566
Batty KT, Ilett KF, Davis TM (2004) Protein binding and alpha: beta anomer ratio of dihydroartemisinin in vivo. Br J Clin Pharmacol 57(4):529–533
Jusko WJ, Milsap RL (1993) Pharmacokinetic principles of drug distribution in saliva. Ann N Y Acad Sci 694:36–47
Posti J (1982) Saliva-plasma drug concentration ratios during absorption: theoretical considerations and pharmacokinetic implications. Pharm Acta Helv 57(3):83–92
Acknowledgments
The authors give their appreciations to the diligent staff at the Medical Clinic at University of Heidelberg. TE thanks Richard Höglund at the Unit for Pharmacokinetics and Drug Metabolism at the University of Gothenburg for his valuable input during the modeling process. Also, appreciations to Dafra Pharma International, Research & Development (Turnhout, Belgium), who supplied the study medication.
Conflicts of interest
The clinical study was supported by H. W. and J. Hector Stiftung, Weinheim, Germany, and Monika-Kutzner-Stiftung, Berlin, Germany. The co-author Antje Blank received personal funding from the Medical Faculty of the University of Heidelberg. The authors further certify that there is no other financial involvement or conflicts of interest regarding the material discussed in the manuscript.
Contribution of authors
T.E.—drug quantitation in plasma and saliva samples, population pharmacokinetic analyses; drafted and finalized manuscript; corresponding author
A.Ä.—senior contribution to the population pharmacokinetic analysis, revised the final manuscript for important intellectual content.
A.B...—contributed to the planning and conduct of study, revised the final manuscript for important intellectual content
C.v.H.—main contributor to the study design and conduct
M.A.—contributed to the study design, interpretation of results, and manuscript development.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement 1
Basic goodness-of-fit plots for artesunate (ARS) in plasma (A, B, C), dihydroartemisinin (DHA) in plasma (D, E, F), and DHA in saliva (G, H, I) using model A and full concentration-time profile data plus sparse sample data. Observed concentrations plotted against population predicted concentrations (A, D, G) and individual predicted concentrations (B, E, H), respectively. Conditional weighted residuals plotted against time after dose (C, F, H). Time axes are truncated to 6.5 h for plasma ARS and salivary DHA, and to 10.5 h for plasma DHA, respectively. Solid lines represent the identity lines and the dashed lines are the locally weighted least square regression lines. All concentrations are represented as transformed values using the natural logarithm. Observed ARS plasma concentrations equal to −5.04 seen in the lower part of plot A and B, represent the BQL data that was imputed as LLOQ/2. (GIF 103 kb)
Supplement 2
Basic goodness-of-fit plots for plasma artesunate (ARS) (A, B, C) and dihydroartemisinin (DHA) (D, E, F) using model B and only full concentration-time profile data. Observed concentrations plotted against population predicted concentrations (A, D) and individual predicted concentrations (B, E), respectively. Conditional weighted residuals plotted against time after dose (C, F). Time axes are truncated to 6.5 and 8.5 h for ARS and DHA, respectively. Solid lines represent the identity lines and the dashed lines are the locally weighted least square regression lines. All concentrations are represented as transformed values using the natural logarithm. Observed ARS plasma concentrations equal to −5.04 seen in the lower part of plot A and B, represent the BQL data that was imputed as LLOQ/2. (GIF 81 kb)
Rights and permissions
About this article
Cite this article
Ericsson, T., Blank, A., von Hagens, C. et al. Population pharmacokinetics of artesunate and dihydroartemisinin during long-term oral administration of artesunate to patients with metastatic breast cancer. Eur J Clin Pharmacol 70, 1453–1463 (2014). https://doi.org/10.1007/s00228-014-1754-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1754-2