Abstract
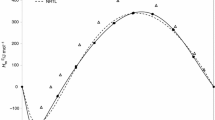
In this work, the new set of the data on the excess heat capacity (CEP ), density (ρ) and excess volumes (VE) for binary system n-propanol + water in the temperature range 278.15–358.15 K are presented. The concentration dependence of CEP was correlated using Redlich–Kister equation. Obtained experimental data of CEP were used to calculate excess enthalpies at various temperatures.






Similar content being viewed by others
References
Demirel Y, Paksot H. Excess heat capacity surfaces for water-alkanol mixtures by the UNIQUAC model. Ind Eng Chem Res. 1995;34:921–7.
Raeva VM, Frolkova AK, Arutyunov BA, Serafimov LA. Concentration dependences of the isobaric heat capacity of binary solutions and its contribution to thermal calculations. Theor Found Chem Eng. 2015;49:667–75.
Malik S, Gupta H, Sharma VK. Excess heat capacities and excess molar enthalpies of the mixtures containing tetrahydropyran, piperidine and cyclic alkanones. J Therm Anal Calorim. 2018;132:1263–75.
Kustov AV, Kudayrova TV, Antonova OA, Smirnova NL. Enthalpies and heat capacities of solution of 3,5-diamino-1,2,4-triazole and 3,5-diamino-1-phenyl-1,2,4-triazole in water. J Therm Anal Calorim. 2019;138:3997–4001.
Gupta H, Malik S, Chandrasekhar M, Sharma VK. Thermodynamic investigations of excess heat capacities of ternary liquid mixtures containing [Bmmim][BF4] + [Bmim][BF4] or [Emim][BF4] + cyclopentanone or cyclohexanone. J Therm Anal Calorim. 2018;131:1653–69.
Niu Q, Zhou C, Zhan Z. Investigation on standard molar enthalpy of combustion, specific heat capacity and thermal behavior of methionine. J Therm Anal Calorim. 2018;132:1805–11.
Gupta H, Malik S, Sharma VK. Excess molar enthalpies for [Bmmim][BF 4] + [Bmim][BF 4] or [Emim][BF 4] + cyclopentanone or cyclohexanone mixtures. J Therm Anal Calorim. 2019;136:1383–94.
Usacheva T, Kabirov D, Beregova D, Gamov G, Sharnin V, Biondi M, et al. Thermodynamics of complex formation between hydroxypropyl-β-cyclodextrin and quercetin in water–ethanol solvents at T = 298.15 K. J Therm Anal Calorim. 2019;138:417–24.
Liu H, Wang C, Zhang J, Luo Q, Sun H, Xu M, et al. The effect of mixture ratio on combustion characteristics of n-propyl alcohol–water binary mixture. J Therm Anal Calorim. 2018;134:2255–64.
Ogawa H, Murakami S. Excess isobaric heat capacities for water + alkanol mixtures at 298.15 K. Thermochim Acta. 1986;109:145–54.
Marcus Y. Water structure enhancement in water-rich binary solvent mixtures. Part II. the excess partial molar heat capacity of the water. J Mol Liq. 2012;166:62–6.
Letyanina I, Tsvetov N, Zvereva I, Samarov A, Toikka A. Excess molar enthalpies for binary mixtures of n-propanol, acetic acid, and n-propyl acetate at 313.15 K and atmospheric pressure. Fluid Phase Equilib. 2014;381:77–82.
Letyanina I, Tsvetov N, Toikka A. Excess molar enthalpies of the ternary mixture n-propanol + acetic acid + water at 313.15 K and atmospheric pressure. Fluid Phase Equilib. 2015;405:150–6.
Letyanina I, Tsvetov N, Toikka A. Application of the UNIFAC models for prediction and description of excess molar enthalpies for binary mixtures of n-propanol, acetic acid, n-propyl acetate, and water. Fluid Phase Equilib. 2016;427:202–8.
Letyanina IA, Tsvetov NS, Zvereva IA, Toikka AM. Measurement and prediction of excess molar enthalpies for ternary mixture n-propanol + acetic acid + n-propyl acetate at 313.15 K. J Therm Anal Calorim. 2016;124:693–9.
Tsvetov N, Letyanina I, Zvereva I, Toikka A. Excess molar enthalpies of the ternary mixture n-propanol + n-propyl acetate + water at 313.15 K. J Therm Anal Calorim. 2018;133:1149–56. https://doi.org/10.1007/s10973-018-7110-5.
Toikka AM, Toikka MA, Pisarenko YA, Serafimov LA. Vapor-liquid equilibria in systems with esterification reaction. Theor Found Chem Eng. 2009;43:129–42.
Toikka AM, Samarov AA, Toikka MA. Phase and chemical equilibria in multicomponent fluid systems with a chemical reaction. Russ Chem Rev. 2015;84:378–92.
Toikka MA, Tsvetov NS, Toikka AM. Splitting of the liquid solution and the compositions of liquid phases in the water-n-propanol-n-propyl acetate system at 293.15, 303.15, and 313.15 K. Theor Found Chem Eng. 2011;45:429–35.
Góralski P, Tkaczyk M. Heat capacities of some liquid α, ω-alkanediamines in the temperature range between (293.15 and 353.15) K. J Chem Eng Data. 2010;55:953–5.
Sharma VK, Kataria J. Topological investigations of excess heat capacities of binary liquid mixtures containing lactams and cycloalkanone. J Mol Liq. 2013;188:210–21.
Sabbah R, Xu-wu A, Chickos JS, Leitão MLP, Roux MV, Torres LA. Reference materials for calorimetry and differential thermal analysis. Thermochim Acta. 1999;331:93–204.
Ginnings DC, Furukawa GT. Heat capacity standards for the range 14 to 1200°K. J Am Chem Soc. 1953;75:522–7.
Frenkel M, Chirico RD, Diky V, Kazakov AF, Muzny CD, Magee JW, Abdulagatov I, Kroenlein K, Magee JW. NIST ThermoData Engine, NIST Standard Reference Database 103b-Pure Compounds, Binary Mixtures, and Chemical Reactions, Version 5. 0, Standard Reference Data Program. Gaithersburg: National Institute of Standards and Technology; 2010.
Redlich O, Kister AT. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948;40:345–8.
Christensen JJ, Hanks RW, Izatt RM. Handbook of heats of mixing. New York: Wiley; 1982.
Acknowledgements
Maria Toikka, Nikita Tsvetov and Anna Sadaeva acknowledge Russian Foundation for Basic Research (Grant 18-33-20138) for the support of the study of thermochemical and excess volume properties of biofuel compounds. The investigations were carried out using the equipment of the Resource Center of Thermogravimetric and Calorimetric Research and Centre for Diagnostics of Functional Materials for Medicine, Pharmacology and Nanoelectronics (Research Park of St. Petersburg State University).
Author information
Authors and Affiliations
Contributions
Nikita Tsvetov carried out the experiments on heat capacity and thermodynamic calculations. Maria Toikka and Anna Sadaeva measured the densities, calculated excess volume and analyzed obtained thermal and volumetric data. Alexander Toikka and Irina Zvereva took part in the general discussion of the results and analysis of the data. All authors contributed to the preparation of the text of the paper.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsvetov, N., Sadaeva, A., Toikka, M. et al. Excess molar heat capacity for the binary system n-propyl alcohol + water in the temperature range 278.15–358.15 K: new data and application for excess enthalpy calculation. J Therm Anal Calorim 142, 1977–1987 (2020). https://doi.org/10.1007/s10973-020-09605-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09605-y