Abstract
Blood plasma and serum are important diagnostic materials as they clearly reflect an individual’s metabolism. The study has evaluated the effect of whole-body cryostimulation (WBC) on the blood serum of professional cross-country skiers. The experiment involved eight athletes (two women and six men) who underwent a series of ten WBC treatments. Aqueous solutions of human blood serum samples before WBC procedures, after one treatment and after a series of ten treatments were measured by means of differential scanning calorimetry (DSC), a relatively novel diagnostic tool. DSC results showed rather little impact of cryostimulation on heat capacity changes accompanying the process of thermal denaturation of blood serum proteins in elite athletes. However, the statistically significant reduction in the intensity of the serum denaturation transition in its low temperature range has been observed after ten WBC treatments. The results have been interpreted by changes in the serum proteome profile, notably in the ratio of ligated to unligated albumin molecules. As a side result, the relationships between the relative change in body fat mass after ten WBC treatments and the levels of alpha2-globulins and beta2-globulins fractions have been found.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cross-country skiing belongs to the group of winter sports. The preparatory period in this group of sports is from spring to autumn [1]. An athlete who trains skiing engages most of his muscles. Skiers should not only have highly anaerobic, but also aerobic power and endurance, because the ski trails have a varied profile [1,2,3,4]. Therefore, it is important to prepare a cross-country skier to continuous changes in the intensity of the effort.
National representatives, elite athletes are subjected to various activities supporting their preparatory process for the start-up season. Whole-body cryostimulation (WBC) is used very often in sports medicine in rehabilitation, treatment of injuries or biological regeneration due to its positive effect on the body of athletes [5,6,7,8,9,10,11,12,13,14]. It has been shown that WBC affects the level of cytokines [5, 6, 11], reduces athletic hemolysis [5, 7, 8], reduces pro-inflammatory responses [5, 8], supports muscle and whole-body regeneration [5, 8, 9, 11, 12], supports the reduction in oxidative stress [5, 10, 14], counteracts the negative effects of exercise overloads [5, 8, 12, 13], supports the treatment of sports injuries [5, 8, 12, 13], prepares the body for higher loads [12] and reduces pain [13]. There are also works in which there were no positive effects of using cryotherapy, and they should not be neglected [5]. Such works should be an incentive for cooperation and further research on the impact of cryostimulation on the human body.
For several years, many attempts have been made to apply the differential scanning calorimetry (DSC) method in various areas of medical diagnostics and sports medicine. DSC profiles of biological fluids can be a source of valuable information about the health of patients as well as athletes [15,16,17,18,19,20,21,22,23,24,25,26,27]. The DSC method was used in patients with multiple disease entities [15,16,17,18,19,20,21,22,23,24]. The branch of research among athletes is just developing [25,26,27]. Further research is needed on various groups of athletes to effectively support sports training and help in the case of problems related to the health of athletes.
The aim of the conducted research was to assess the impact of cryostimulation treatments on the course of the process of thermal denaturation of proteins contained in blood sera of elite athletes, well trained in cross-country skiing.
Materials and methods
Participants
Eight athletes (two women and six men) well trained in cross-country skiing participated in this study. Athletes participating in our study belong to the national team and take part in competitions around the world. The study was performed during the transient phase of the annual training plan. A group of these athletes has undergone a series of ten cryostimulation treatments. Characteristics of the group of athletes before the treatments (mean ± SD) were age 22.8 ± 2.9 year, mass 73.7 ± 9.6 kg, height 176.7 ± 11.0 cm, BMI 23.6 ± 1.4 kg m−2, skeletal muscle mass 36.8 ± 7.0 kg, fat mass 9.3 ± 2.9 kg, percentage of body fat 13.1 ± 5.8%. After a series of ten cryostimulation treatments, athletes were again characterized and two of these parameters changed: fat mass to 8.2 ± 3.6 kg and percentage of body fat to 11.8 ± 6.7%.
Blood samples were taken from athletes before the beginning of cryostimulation, after the first treatment and after a series of ten cryostimulation treatments. Blood collection, of which the sera for the calorimetric study were obtained, took place the next day after completing one or ten WBC treatments. All blood samples were taken in the morning. A number of biochemical tests were performed on samples from athletes, including electrophoresis and creatine kinase assay.
All athletes were informed about the purpose and the nature of the research before giving their written consent to participate in the experiment. The studies were performed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the Jerzy Kukuczka Academy of Physical Education in Katowice (Certificate of Approval No. 2/2016).
Whole-body cryostimulation
Cryostimulation (WBC) procedure was carried out in the cryo-chamber located in Upper Silesian Center of Medicine and Rehabilitation in Katowice. The treatment sessions were held in the afternoons, 5 days a week, from Monday to Friday for 2 weeks. The athletes entered the chamber in groups of five persons. Each cryostimulation session lasted 3 min (− 130 °C). Entry to the cryo-chamber was preceded by a 30-s adaptation period in the vestibule at a temperature of − 60 °C, from which the athletes went further to the proper chamber, where they moved slowly in a circle, one after the other, without mutual contact, no additional movement or talking. After a minute, a change in the direction of motion was recommended. Contact with the participants was maintained via a camera in the room and voice contact.
Preparation of serum samples
Blood serum samples were prepared using the standard method and kept frozen at − 20 °C until the measurement. To prepare serum solutions, we thawed the serum samples at room temperature for 10 min. Then, 20-fold diluted solution was prepared using the degassed water (in a ratio of 0.125 mL serum and 2.375 mL degassed water using an automatic pipette to obtain 2.5 mL total solution). The pH value of the tested aqueous serum solutions was about 7.0 ± 0.5.
The DSC method
Differential scanning calorimetry (DSC) measurements of aqueous serum solutions were made using the VP-DSC microcalorimeter (MicroCal Co., Northampton, MA). Two scans were obtained for each sample in the temperature range 20–100 °C with a heating rate of 1 °C min−1 and a pressure of about 1.7 × 105 Pa. All raw DSC curves were corrected for the water–water instrumental line. The next steps were normalization for the gram mass of protein and subtracting the linear baseline. The following thermodynamic parameters: temperatures (T1, T2, T3) and excess heat capacities (Cp1, Cp2, Cp3) of local maxima, the enthalpy (∆H) of thermal denaturation of serum as the area under the endothermic peak and the width of peak in its half height (HHW), were determined.
Statistics
Statistical analysis was carried out with the Statistica 13.1 program. After checking for normal distribution (tested by Schapiro–Wilk test), ANOVA with repeated measurements has been used. Mauchly’s test for sphericity was included as a part of the procedure. When the ANOVA with repeated measurements was statistically significant, the post hoc Tukey’s test was performed. The level of statistical significance was set at p < 0.05. The T test for the dependent groups was performed to compare biochemical parameters before and after ten WBC treatments. Linear regression and Pearson’s correlation coefficient (r) were used to evaluate the relationships between protein fractions, biochemical and thermodynamic parameters of blood serum.
Results and discussion
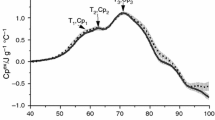
Thermal denaturation of human blood serum is observed in DSC measurements as the complex endothermic transition in the temperature range of 40 °C to 90 °C. It is the result of denaturation of the constituent proteins and represents the weighted sum of denaturation profiles of the individual proteins within serum. This transition is irreversible. Usually, two or three main peaks can be distinguished in the DSC profile of healthy human blood serum, depending on the solvent used [14,15,16,17,18,19,20,21,22,23,24,25,26].
In aqueous solution, the first peak with the contribution mainly from nonligated albumin is observed at about 57 °C. The second local maximum (not always visible), mainly derived from haptoglobin, appears at about 62 °C. In buffer solutions (pH 7.4), these two peaks merge into one centered at about 63 °C. The contribution from immunoglobulins reveals itself around 70 °C in both kinds of serum/plasma solutions. Each of these three peaks still contains small contributions from other serum proteins (alpha1-globulin, alpha2-globulin, beta1-globulin, beta2-globulin).
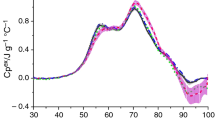
Figure 1 shows the averaged DSC curves of sera from each stage of the experiment: before WBC treatments (0 WBC), after one WBC treatment (1 WBC) and after series of ten WBC treatments (10 WBC). In all curves, we can observe local maxima at temperatures of about 57 °C, 62 °C and 70 °C. The peak at about 70 °C dominates over the less intense other two. The mean thermodynamic parameters for denaturation transition of athlete’s serum are listed in Table 1. The HHW at each stage of the experiment was practically the same, about 25 °C.
DSC curves of blood serum collected from athletes before using WBC and after one treatment are very similar to each other, and slight differences between them are within the limits of experimental error. The differences are visible by comparing these curves with curve corresponding to serum collected after a series of ten WBC treatments. In the case of the first local maximum, after ten WBC treatments, its intensity is lower than before WBC and after one WBC treatment. The results of ANOVA with repeated measure (p = 0.04) have indicated statistically significant differences between mean values of Cp1 in three considered stages of the experiment. Post hoc Tukey’s test has shown statistically significant difference between Cp1 without WBC and after ten WBC treatments (p = 0.037). Results shown in Table 1 suggest also a decrease in ∆H value after completing a total of ten whole-body therapy sessions, but this trend is not statistically significant.
A decrease in the intensity of the first local maximum (Cp1) would suggest a decrease in the level of serum albumin in participants of this study. However, results from the electrophoresis show that this is a mistaken idea. Table 2 presents the average value of total protein and the average percentage of the most abundant proteins fractions in serum at each stage of the experiment. Results shown in this table suggest an increase in the percentage of serum albumin after cryostimulation treatments. Actually, ANOVA with repeated measure (p = 0.03) has indicated statistically significant differences between mean percentage of albumin in three stages of experiment; in particular, post hoc Tukey’s test has shown significant difference between percentage of albumin before WBC and after ten WBC treatments (p = 0.025).
The exposure to WBC results in an elevated metabolism in order to increase heat production to protect the core of the body. The blood is enriched with nutrients, enzymes and oxygen intensively. Thus, more ligands are available to bind to albumin. This may lead to an increase in the ratio of ligated to unligated albumin molecules. A decrease of % content of nonligated albumin fraction, in spite of the increase in total albumin level after ten WBC, can lead to a decrease in Cp1 value. We think that such perturbation of the shape of serum denaturation transition after a series of WBC treatments, corresponding to ligand interactions with serum albumin, would be visible regardless of belonging to a group of athletes, due to an elevated level of endogenous ligand components in serum after WBC stimulation.
It seems noteworthy that after ten WBC treatments, negative correlation between percentage of albumin and ∆H value (r = − 0.9 and p = 0.008) has been found (Fig. 2). This correlation shows that after ten WBC treatments, lower enthalpy of serum denaturation has been observed for athletes with higher percentage content of albumin in serum. As cryostimulation helps create extra oxygen in the bloodstream, it can result in increasing fraction of oxidized form of serum albumin. This form of albumin is characterized by lower enthalpy of denaturation than the nonoxidized one [28]. Higher percentage of albumin after ten WBC treatments entails higher level of oxidized albumin molecules and lower ∆H.
Results shown in Table 2 suggest also a decrease in total protein value after completing a total of ten whole-body therapy sessions but this change was not statistically significant. The amount of gamma globulins has remained at a similar level despite cryostimulation treatments.
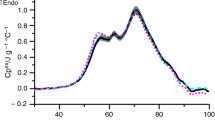
Changes in blood serum reflect an individual’s metabolism. DSC thermal profiles of samples gathered in this study have shown that not everyone responded to cryostimulation in the same way. Figure 3 shows DSC curves for the athlete whose reaction was different from the one indicated by mean DSC curves presented in Fig. 1. The decrease in Cp1 and ∆H values after WBC is not observed in this case. The main effect clearly visible in Fig. 3 concerns the second local maximum (T2, Cp2). It is not present before the WBC treatments, reveals itself after one WBC treatment and becomes more intense after ten WBC treatments. The enhancement of this maximum may indicate the development of inflammation or injury because this peak shows the presence of an acute phase protein, haptoglobin. This protein belongs to alpha2-globulins whose percentage content began to increase after just one WBC treatment and remained elevated after ten WBC according to electrophoretic results for this athlete. The elevated value of blood creatine kinase (CK), which is a sensitive index of skeletal muscle injuries [29], has been also found for this cross-country skier. It may suggest that he has suffered injury during his daily trainings.
A side result of our studies is the suggestion that WBC has the effect of reducing the amount of body fat. This is not a quite new discovery that cryostimulation can help in mass loss. To reheat the body and to maintain body temperature after WBC, the metabolism is accelerated, which allows increased calorie burning. A controlled cold stimulation is used for destruction of fat cells. Currently, cryolipolysis is widely used to achieve mass loss effects [30].
Despite the small group in the current study, the T test for dependent samples showed statistically significant decrease (p = 0.022) of the mean value of fat mass (from 9.3 ± 2.9 kg before treatments to 8.2 ± 3.6 kg after ten WBC treatments). A similar result was obtained for % fat content (p = 0.026). Additionally, a high positive correlation (r = 0.74 and p = 0.037) between the change in Cp1 value (diff Cp1 = Cp1 before WBC − Cp1 after ten WBC) and the corresponding relative change in body fat mass after ten WBC treatments has been found (Fig. 4). It suggests that the decrease in relative fat mass observed in most cases after WBC is accompanied by the decrease in Cp1 parameter of serum denaturation transition. The decrease in Cp1 has been suggested to be associated with the change in albumin fractions ratio. The question arises whether this is the only possible explanation. The other important issue seems to be the relationship between changes in fat mass and the content of particular protein fractions in athlete’s blood serum. So, the next step of the analysis was an attempt to find such connections. According to Geyer et al. results, the mass loss changes multiple components of the plasma proteome [31, 32]. They reported a significant increase in albumin and a decrease in some proteins of special clinical interest, mainly apolipoproteins and markers of inflammation, concomitantly with mass loss.
In this study, the high, statistically significant correlations have been found between the relative change in body fat mass after ten WBC treatments and the change (difference between the concentration of considered protein fraction before WBC and after ten WBC treatments) in the level of alpha2-globulins (r = 0.82 and p = 0.023) and beta2-globulins (r = 0.91 and p = 0.004). Both of these correlations are positive. This suggests a down-regulation of proteins from these fractions in response to the loss of fat mass. The alpha2-globulin fraction includes proteins such as alpha2-macroglobulin, haptoglobin and ceruloplasmin, while to the beta2-globulin fraction belong beta2-microglobulin and beta-lipoproteins.
Recently, Polidori et al. [33] have shown that the body fat content affects the effectiveness of whole-body cryotherapy. They suggested that women are more sensitive to the extreme cold than men due to the greater inner thermal resistance connected with a higher concentration of body fat percentage. The difference in adaptive thermogenesis between lean and obese people has been reported by Wijers et al. [34]. Their studies have revealed that most obese subjects counteracted the cold by increasing insulation, while the lean subjects predominantly increased energy expenditure. However, they found large interindividual differences in the relative contribution of both mechanisms.
Only two women participated in our study. Their body fat percentage was higher than for all male participants and practically did not change after the WBC session. Therefore, females’ response to WBC relating to fat mass was different compared to male. Cross-country skiers are rather slim people, and they have little fat and a lot of skeletal muscles. So, they react to extreme cold temperatures mainly by increase in energy expenditure which leads to a caloric deficit and next to a reduction in fat. This effect seems to be undesirable for athletes who are exposed to changing environmental conditions taking part in competitions held in different parts of the world.
Conclusions
DSC results indicate that whole-body cryostimulation affects blood serum of professional cross-country skiers. The intensity of the endothermic transition connected with serum denaturation decreases after the cryostimulation cycle, particularly in the low temperature range of this transition. The decrease in Cp1 mean value after the whole series of ten WBC treatments has been found statistically significant. As this low-temperature region of the complex transition corresponds principally to the denaturation of unligated albumin, this result has indicated a decrease in this form of albumin. With regard to the results of electrophoretic assay that total albumin level has increased after the ten WBC treatments, the possible explanation is that the decrease in the number of ligands-free albumin molecules is accompanied by an increased fraction of albumin with bound ligands, including oxidized albumin. An increasing fraction of oxidized form of serum albumin is in line with the fact that cryostimulation helps create extra oxygen in the bloodstream. The higher level of oxidized albumin molecules for which enthalpy of denaturation is lower than for nonoxidized one is in accordance with a decrease in ∆H (although not statistically significant) after ten WBC treatments.
Unexpectedly, statistically significant, high positive correlation between the change in Cp1 and the relative change in body fat mass after ten WBC treatments has been found. Similar correlations have occurred between the relative change in body fat mass after ten WBC treatments and the corresponding changes in alpha2-globulins and beta2-globulins levels, suggesting the down-regulation of proteins from these fractions in response to the loss of fat mass as a result of cryostimulation. A larger number of blood serum samples would be required for future verification of this conclusion.
References
Solli GS, Tønnessen E, Sandbakk Ø. The training characteristics of the world’s most successful female cross-country skier. Front Physiol. 2017. https://doi.org/10.3389/fphys.2017.01069.
McGawley K, Holmberg H-Ch. Aerobic and anaerobic contributions to energy production among junior male and female cross-country skiers during diagonal skiing. Int J Sports Physiol Perform. 2014;9:32–40.
Sandbakk Ø, Holmberg HC. Physiological capacity and training routines of elite cross-country skiers: approaching the upper limits of human endurance. Int J Sports Physiol Perform. 2017;12:1003–11.
Pellegrini B, Zoppirolli C, Bortolan L, Holmberg HC, Zamparo P, Schena F. Biomechanical and energetic determinants of technique selection in classical cross-country skiing. Hum Mov Sci. 2013;32:1415–29.
Lombardi G, Ziemann E, Banfi G. Whole-body cryotherapy in athletes: from therapy to stimulation. An updated review of the literature. Front Physil. 2017. https://doi.org/10.3389/fphys.2017.00258.
Lubkowska A, Szyguła Z, Chlubek D, Banfi G. The effect of prolonged whole-body cryostimulation treatment with different amounts of session on chosen pro- and anti-inflammatory cytokines levels in healthy men. Scand J Clin Lab Invest. 2011;71:419–25.
Banfi G, Melegati G, Barassi A, Melzi d’Eril G. Beneficial effects of the whole-body cryotherapy on sport haemolysis. J Hum Sport Exerc. 2009;2:189–93.
Banfi G, Lombardi G, Colombini A, Melegati G. Whole-body cryotherapy in athletes. Sports Med. 2010;40(6):509–17.
Ferreira-Junior JB, Bottaro M, Loenneke JP, Vieira A, Vieira Carlos A, Bemben MG. Could whole-body cryotherapy (below − 100 °C) improve muscle recovery from muscle damage? Front Physiol. 2014. https://doi.org/10.3389/fphys.2014.00247.
Stanek A, Sieroń-Stołtny K, Romuk E, Cholewka A, Wielkoszyński T, Cieślar G, Kwiatek S, Sieroń A, Kawczyk-Krupka A. Whole-body cryostimulation as an effective method of reducing oxidative stress in healthy men. Adv Clin Exp Med. 2016;25(6):1281–91.
Ziemann E, Olek RA, Kujach S, Grzywacz T, Antosiewicz J, Garsztka T, Laskowski R. Five-day whole-body cryostimulation, blood inflammatory markers, and performance in high-ranking professional tennis players. J Athl Train. 2012;47(6):664–72.
Jonak A, Skrzek A. Krioterapia w odnowie biologicznej sportowców—przegląd badań. Cryotherapy in athletes’ biological regeneration—review. Acta Bio-Optica et Informatica Medica. 2009;15:319–21.
Rossato M, De Souza Bezerra E, de Ceselles Seixas da Silva DA, Avila Santana T, Rafael Malezam W, Carpes FP. Effects of cryotherapy on muscle damage markers and perception of delayed onset muscle soreness after downhill running: a pilot study. Revista Andaluza de Medicina del Deporte. 2015;8(2):49–53.
Stanek A, Cholewka A, Wielkoszyński T, Romuk E, Sieroń A. Decreased oxidative stress in male patients with active phase ankylosing spondylitis who underwent whole-body cryotherapy in closed cryochamber. Oxid Med Cell Longev. 2018. https://doi.org/10.1155/2018/7365490.
Garbett NC, Miller JJ, Jenson AB, Chaires JB. Calorimetry outside the box: a new window into the plasma proteome. Biophys J. 2008;94:1377–83.
Velazquez-Campoy A, Vega S, Sanchez-Gracia O, Lanas A, Rodrigo A, Kaliappan A, Hall MB, Nguyen TQ, Brock GN, Chesney JA, Garbett NC, Abian O. Thermal liquid biopsy for monitoring melanoma patients under surveillance during treatment: a pilot study. BBA Gen Subj. 2018;1862:1701–10.
Garbett NC, Mekmaysy CS, Helm CW, Jenson AB, Chaires JB. Differential scanning calorimetry of blood plasma for clinical diagnosis and monitoring. Exp Mol Pathol. 2009;86:186–91.
Garbett NC, Brock GN. Differential scanning calorimetry as a complementary diagnostic tool for the evaluation of biological samples. Biochimica et Biophysica Acta. 2016;1860:981–9.
Todinova S, Krumova S, Danailova A, Petkova V, Guenova M, Mihaylov G, Gartcheva L, Taneva SG. Calorimetric markers for monitoring of multiple myeloma and Waldenström’s macroglobulinemia patients. Eur Biophys J. 2018;47:549–59.
Michnik A, Sadowska-Krępa E, Cholewa J, Schisler I, Kiełboń A, Drzazga Z. Differential scanning calorimetry study of early and advanced stages in Parkinson’s disease using human blood serum. Thermochim Acta. 2018;662:64–8.
Kędra-Królik K, Chmielewska I, Michnik A, Zarzycki P. Blood serum calorimetry indicates the chemotherapeutic efficacy in lung cancer treatment. Sci Rep. 2017. https://doi.org/10.1038/s41598-017-17004-x.
Zapf I, Fekecs T, Ferencz A, Tizedes G, Pavlovics G, Kalman E, Lőrinczy D. DSC analysis of human plasma in breast cancer patients. Thermochim Acta. 2011;524:88–91.
Fekecs T, Zapf I, Ferencz A, Lőrinczy D. Differential scanning calorimetry (DSC) analysis of human plasma in melanoma patients with or without regional lymph node metastases. J Therm Anal Calorim. 2012;108:149–52.
Farkas P, Könczöl F, Lőrinczy D. New possibilities of application of DSC as a new clinical diagnostic method. J Therm Anal Calorim. 2018;133:579–89.
Michnik A, Drzazga Z, Poprzęcki S, Czuba M, Kempa K, Sadowska-Krępa E. DSC serum profiles of sportsmen. J Therm Anal Calorim. 2013;113:365–70.
Michnik A, Sadowska-Krępa E, Domaszewski P, Duch K, Pokora I. Blood serum DSC analysis of well-trained men response to CrossFit training and green tea extract supplementation. J Therm Anal Calorim. 2017;130:1253–62.
Michnik A, Drzazga Z, Schisler I, Poprzęcki S, Czuba M. Diversity in athlete’s response to strength effort in normobaric hypoxia. J Therm Anal Calorim. 2018;134:633–41.
Gorobets MG, Wasserman LA, Vasilyeva AD, Bychkova AV, Pronkin PG, Bugrova AE, Indeykina MI, Shilkina NG, Konstantinova ML, Kononikhin AS, Nikolaev EN, Rosenfeld MA. Modification of human serum albumin under induced oxidation. Dokl Biochem Biophys. 2017;474(1):231–5.
Takagi Y, Yasuhara T, Gomi K. Creatine kinase and its isozymes. Rinsho Byori Jpn J Clin Pathol. 2001;116:52–61.
Meyer PF, Valentim da Silva RM, Oliveira G, Azevedo da Silva Tavares M, Medeiros ML, Andrada CP, de Araujo Neto LG. Effects of cryolipolysis on abdominal adiposity. Case Rep Dermatol Med. 2016. https://doi.org/10.1155/2016/6052194.
Geyer PE, Kulak NA, Pichler G, Holdt LM, Teupser D, Mann M. Plasma proteome profiling to assess human health and disease. Cell Syst. 2016;2:185–95.
Geyer PE, Wewer Albrechtsen NJ, Tyanova S, Grassl N, Jepsen EW, Lundgren J, Madsbad S, Holst JJ, Torekov SS, Mann M. Proteomics reveals the effects of sustained weight loss on the human plasma proteome. Mol Syst Biol. 2016. https://doi.org/10.15252/msb.20167357.
Polidori G, Cuttell S, Hammond L, Langdon D, Legrand F, Taiar R, Boyer FC, Costello JT. Should whole body cryotherapy sessions be differentiated between women and men? A preliminary study on the role of the body thermal resistance. Med Hypotheses. 2018;120:60–4.
Wijers SLJ, Saris WHM, van Marken Lichtenbelt WD. Cold-induced adaptive thermogenesis in lean and obese. Obes J. 2010;18:1092–9.
Funding
The project has been financed by the grant Ministry of Science and Higher Education/Nr 0050/RS4/2016/54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Duch, K., Michnik, A., Pokora, I. et al. Whole-body cryostimulation impact on blood serum thermal denaturation profiles of cross-country skiers. J Therm Anal Calorim 138, 4505–4511 (2019). https://doi.org/10.1007/s10973-019-08766-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08766-9