Abstract
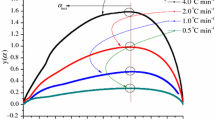
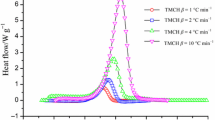
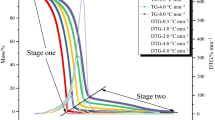
Tert-butylperoxy-2-ethylhexyl ester (TBEC) is a typical organic peroxide that is used in pharmaceuticals, materials, intermediates or as a polymerization initiator, and it has a large amount of consumption. We examined the thermal risks of TBEC during storage and transport by using differential scanning calorimetry and thermal activity monitor III to investigate its thermal stability and thermal hazards. Non-isothermal and isothermal experiments were used to calculate the apparent activation energy (E(α)), compare the calculated kinetic data, pre-exponential factor, and other parameters, and infer the thermal behaviour of TBEC under pseudo-adiabatic conditions. According to the calculation results, the E(α) under non-isothermal conditions calculated through the Friedman isothermal kinetic method ranged from 91.47 to 177.35 kJ mol−1. E(α) under isothermal conditions ranged from 157.18 to 143.28 kJ mol−1. TBEC was also tested under pseudo-adiabatic conditions using vent sizing package 2 to investigate uncontrolled thermal runaway. The calculated self-accelerating decomposition temperature was 34.0 °C. Our results provide an insight into the thermal decomposition of TBEC and supply reliable data for its storage and transport processes.









Similar content being viewed by others
Abbreviations
- A :
-
Frequency factor (M1–n s−1)
- C p :
-
Specific heat capacity at constant pressure (kJ kg−1 K−1)
- E(α):
-
Apparent activation energy (kJ mol−1)
- f(α):
-
Kinetic function depends on conversions (dimensionless)
- (dP/dt)max :
-
Maximum pressure rise rate (psig min−1)
- (dT/dt)max :
-
Maximum temperature rise rate (°C min−1)
- t :
-
Time (s)
- T o :
-
Exothermic onset temperature (°C or K)
- T p1 :
-
Exothermic peak temperature of the first peak in DSC curves (°C or K)
- T p2 :
-
Exothermic peak temperature of the second peak in DSC curves (°C or K)
- SADT :
-
Self-accelerating decomposition temperature (°C or K)
- TMR ad :
-
Time to maximum rate under pseudo-adiabatic conditions (h or day)
- TMR iso :
-
Time to maximum rate under isothermal conditions (h or day)
- α :
-
Reaction conversion (dimensionless)
- β :
-
Heating rate (°C min−1)
- Ф :
-
Thermal inertia factor (dimensionless)
- ΔH d :
-
Heat of decomposition (J g−1)
- W p :
-
Peak power (W g−1)
References
Chen CC, Duh YS, Shu CM. Thermal polymerization of uninhibited styrene investigated by using microcalorimetry. J Hazard Mater. 2009;163:1385–90.
Liu SH, Hou HY, Chen JW, Lin SY, Shu CM. Effects of thermal runaway hazard for three organic peroxides conducted by acids and alkalines with DSC, VSP2, and TAM III. Thermochim Acta. 2013;566:226–32.
Globally Harmonized System of Classification and Labelling of Chemicals (GHS). United Nations Publications, New York (USA) and Geneva (Switzerland) 2019.
He J. Study on thermal decomposition analysis of organic peroxides. Nanjing: Nanjing University of Science & Technology; 2008.
Chen WC, Shu CM. Prediction of thermal hazard for TBPTMH mixed with BPO through DSC and isoconversional kinetics analysis. J Therm Anal Calorim. 2016;126:1–9.
Kao CS, Hu KH. Acrylic reactor runaway and explosion accident analysis. J Loss Prevent Proc Ind. 2002;15:213–22.
Lee RP, Hou HY, Tseng JM, Chang MK, Shu CM. Reactive incompatibility of DTBP mixed with two acid solutions. J Therm Anal Calorim. 2008;93:269–74.
Liu SH, Chen YC, Hou HY. Thermal runaway hazard studies for ABVN mixed with acids or alkalines by DSC, TAM III, and VSP2. J Therm Anal Calorim. 2015;122:1107–16.
Tseng JM, Lin CP, Huang ST, Hsu J. Kinetic and safety parameters analysis for 1,1,-di-(tert-butylperoxy)-3,3,5-trimethylcyclohexane in isothermal and non-isothermal conditions. J Hazard Mater. 2011;192:1427–36.
Streltsov DR, Buzin AI, Dmitryakov PV, Bessonova NP, Kamasa P, Ivanov DA, Chvalun SN. A study of p-xylylene polymerization kinetics by isoconversional analysis. Thermochim Acta. 2013;573:175–80.
Zhang Y, Chung YH, Liu SH, Shu CM, Jiang JC. Analysis of thermal hazards of O,O-dimethyl phosphoramidothioate by DSC, TG, VSP2, and GC/MS. Thermochim Acta. 2017;652:69–76.
Tseng JM, Chang YY, Su TS, Shu CM. Study of thermal decomposition of methyl ethyl ketone peroxide using DSC and simulation. J Hazard Mater. 2007;142:765–70.
Whitmore MW, Wilberforce JK. Use of the accelerating rate calorimeter and the thermal activity monitor to estimate stability temperatures. J Loss Prevent Proc Ind. 1993;6:95–102.
Wang YW, Duh YS, Shu CM. Thermal runaway hazards of tert-butyl hydroperoxide by calorimetric studies. J Therm Anal Calorim. 2009;95:553–7.
Tseng JM, Hsieh TF, Chang YM, Yang YC, Chen LY, Lin CP. Prediction of thermal hazard of liquid organic peroxides by non-isothermal and isothermal kinetic model of DSC tests. J Therm Anal Calorim. 2012;109:1095–103.
Nogueira TR, Lona LMF, McManus NT, Vivaldo-Lima EA. Penlidis, nitroxide-mediated radical copolymerization of styrene and divinylbenzene: increased polymerization rate by using TBEC as initiator. J Mater Sci. 2010;45:1878–84.
Wang TS, Liu SH, Qian XM, You ML, Chou WL, Shu CM. Isothermal hazards evaluation of benzoyl peroxide mixed with benzoic acid via TAM III test. J Therm Anal Calorim. 2013;113:1625–31.
Tseng JM, Liu MY, Chen SL, Hwang WT, Gupta JP, Shu CM. Runaway effects of nitric acid on methyl ethyl ketone peroxide by TAM III tests. J Therm Anal Calorim. 2009;96:789–93.
Roduit B, Borgeat Ch, Berger B, Folly P, Alonso B, Aebischer JN. The prediction of thermal stability of self-reactive chemicals. J Therm Anal Calorim. 2005;80(1):91–102.
Roduit B, Xia L, Folly P, Berger B, Mathieu J, Sarbach A, Andres H, Ramin M, Vogelsanger B, Spitzer D, Moulard H, Dilhan D. The simulation of the thermal behavior of energetic materials based on DSC and HFC signals. J Therm Anal Calorim. 2008;93:143–52.
Wei C, Saraf SR, Rogers WJ, Mannan MS. Thermal runaway reaction hazards and mechanisms of hydroxylamine with acid/base contaminants. Thermochim Acta. 2004;421:1–9.
Wang YW, Duh YS, Shu CM. Evaluation of adiabatic runaway reaction and vent sizing for emergency relief from DSC calorimetry. Ind Eng Chem Res. 2001;40:1125–32.
Mettler Company. DSC 821e Operation instructions. Switzerland 2017.
Roduit B, Borgeat Ch, Berger B, Folly P, Andres H, Schädeli U, Vogelsanger B. Up-scaling of DSC data of high energetic materials. J Therm Anal Calorim. 2006;85:195–202.
Das M, Shu CM. A green approach towards adoption of chemical reaction model on 2,5-dimethyl-2,5-di-(tert-butylperoxy) hexane decomposition by differential isoconversional kinetic analysis. J Hazard Mater. 2016;301:222–32.
Berčič G. The universality of Friedman’s isoconversional analysis results in a model-less prediction of thermodegradation profiles. Thermochim Acta. 2017;650:1–7.
Streltsov DR, Buzin AI, Dmitryakov PV, Kamasa P, Ivanov DA, Chvalun SN. A study of p-xylylene polymerization kinetics using high-vacuum in situ, differential scanning calorimetry. Thermochim Acta. 2016;643:65–72.
AKTS AG. http://www.akts.com (AKTS-Thermokinetics software).
Lin CP, Tseng JM, Chang YM, Chang YM, Cheng YC, Lin HY, Chien CY. Green thermal analysis for predicting thermal hazard of storage and transportation safety for tert-butyl peroxybenzoate. J Loss Prevent Proc Ind. 2012;25:1–7.
Liu SH, Hou HY, Shu CM. Thermal hazard evaluation of the autocatalytic reaction of benzoyl peroxide using DSC and TAM III. Thermochim Acta. 2015;605:68–76.
Li XR, Koseki H. Study on the early stage of runaway reaction under adiabatic conditions. J Loss Prevent Proc Ind. 2005;18:455–9.
Askonas CF, Burelbach JP, Leung JC. The versatile VSP2: a tool for adiabatic thermal analysis and vent sizing applications. In: North American Thermal Analysis Society, 28th annual conference, Orlando, Florida, USA, 4–6 Oct 2000.
Wu SH, Shyu ML, Yet-Pole I, Chi JH, Shu CM. Evaluation of runaway reaction for dicumyl peroxide in a batch reactor by DSC and VSP2. J Loss Prevent Proc Ind. 2009;22:721–7.
Liu XH, Zhu SB, Zhu XZ, Wu XL. Hazard assessment of hydrogen peroxide with polyphosphonic acid by vent sizing package 2. Proc Eng. 2014;84:427–35.
Wu SH, Wu JY, Wu YT, Lee JC, Huang YH, Shu CM. Explosion evaluation and safety storage analyses of cumene hydroperoxide using various calorimeters. J Therm Anal Calorim. 2013;111:669–75.
Gao YJ, Xue Y, Lǚ ZG, Wang ZH, Chen Q, Shi N, Sun F. Self-accelerating decomposition temperature and quantitative structure–property relationship of organic peroxides. Process Saf Envrion Prot. 2015;94:322–8.
Kossoy AA, Sheinman IY. Comparative analysis of the methods for SADT determination. J Hazard Mater. 2007;142:626–38.
Shen SJ, Wu SH, Chi JH, Lin CC, Horng JJ, Shu CM. Simulation of solid thermal explosion and liquid thermal explosion of dicumyl peroxide using calorimetric technique. Simul Model Pract Theory. 2011;19:1251–7.
Wu SH, Chou HC, Pan RN, Huang YH, Horng JJ, Chi JH, Shu CM. Thermal hazard analyses of organic peroxides and inorganic peroxides by calorimetric approaches. J Therm Anal Calorim. 2012;109:355–64.
Roduit B, Hartmann M, Folly P, Sarbach A, Dejeaifve A, Dobson R, Kurko K. Kinetic analysis of solids of the quasi-autocatalytic decomposition type: SADT determination of low-temperature polymorph of AIBN. Thermochim Acta. 2018;665:119–26.
Stoessel F. Thermal safety of chemical processes: risk assessment and process design. New York: Wiley; 2008. p. 311–34.
Acknowledgements
The authors are grateful for the financial support of the National Key Research and Development Program (2016YFC0801500), Major Program of the National Natural Science Foundation of China (2143-6006), Major Projects of the Natural Science Research for Colleges in Jiangsu Province (17KJA620002), Priority Academic Program Development of the Jiangsu Higher Education Institutions, and experimental assistance from the Process Safety and Disaster Prevention Laboratory in Taiwan.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, CY., Lin, WC., Pan, XH. et al. Thermal risk assessment of tert-butylperoxy-2-ethylhexyl carbonate for storage and transport. J Therm Anal Calorim 138, 2891–2900 (2019). https://doi.org/10.1007/s10973-019-08552-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08552-7