Abstract
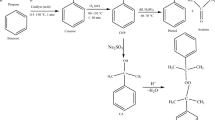
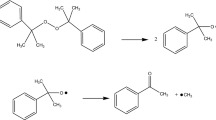
Plenty of thermal explosions and runaway reactions of cumene hydroperoxide (CHP) were described from 1981 to 2010 in Taiwan. Therefore, a thermal explosion accident of CHP in oxidation tower in 2010 in Taiwan was investigated because of piping breakage. In general, high concentration of CHP for thermal analysis using the calorimeter is dangerous. Therefore, a simulation method and a kinetic parameter were used to simulate thermal hazard of high concentrations of CHP only by the researcher. This study was applied to evaluate thermal hazard and to analyze storage parameters of 80 and 88 mass% CHP using three calorimeters for the oxidation tower, transportation, and 50-gallon drum. Differential scanning calorimetry (DSC) (a non-isothermal calorimeter), thermal activity monitor III (TAM III) (an isothermal calorimeter), and vent sizing package 2 (VSP2) (an adiabatic calorimeter) were employed to detect the exothermic behavior and runaway reaction model of 80 and 88 mass% CHP. Exothermic onset temperature (T 0), heat of decomposition (ΔH d), maximum temperature (T max), time to maximum rate under isothermal condition (TMRiso) (as an emergency response time), maximum pressure (P max), maximum of self-heating rate ((dT/dt)max), maximum of pressure rise rate ((dP/dt)max), half-life time (t 1/2), reaction order (n), activation energy (E a), frequency factor (A), etc., of 80 and 88 mass% CHP were applied to prevent thermal explosion and runaway reaction accident and to calculate the critical temperature (T c). Experimental results displayed that the n of 80 and 88 mass% CHP was determined to be 0.5 and the E a of 80 and 88 mass% CHP were evaluated to be 132 and 134 kJ mol−1, respectively.













Similar content being viewed by others
Abbreviations
- A :
-
Frequency factor, sec−1 M1−n
- C p :
-
Liquid specific heat at constant pressure, kJ kg−1 °C−1
- C 0 :
-
Initial concentration, mol L−1
- E a :
-
activation energy, kJ mol−1
- K :
-
Pre-exponential factor, s−1
- M :
-
Mass of reactant, g
- P max :
-
Maximum pressure during overall reaction, psig
- Q :
-
Calorific capacity, J g−1
- \( \dot{Q} \) :
-
Heat flow, W g−1
- R :
-
Gas constant, 8.314 J mol−1 K−1
- S :
-
Wetted surface area, m2
- SADT:
-
Self-accelerating decomposition temperature, °C
- T :
-
Temperature, °C
- T A :
-
Final adjusted temperature, K
- T A0 :
-
Initial adjusted temperature, K
- T f :
-
Final temperature, °C
- T M :
-
Final measured temperature, K
- T max :
-
Maximum temperature during overall reaction, °C
- T M0 :
-
Initial measured temperature, K
- T NR :
-
Temperature of no return, °C
- T wall :
-
Temperature on the wall, °C
- TMRad :
-
Time to maximum rate under adiabatic system, min, hr
- U :
-
Heat transfer coefficient, kJ min−1 m−2 K−1
- a:
-
Vessel wetted surface area, m2
- k i :
-
Rate at stage i, s−1
- m :
-
Mass of reactor, kg
- n :
-
Order of reaction, dimensionless
- α:
-
Degree of conversion, dimensionless
- β:
-
Heating rate, °C min−1
- λ:
-
Heat conductivity, J ms K−1
- ϕ:
-
Thermal inertia, dimensionless
- △H d :
-
Heat of decomposition, J g−1
- (dT/dt):
-
Self-heating rate, °C min−1
- (dT/dt)A :
-
Actual self-heating rate, °C min−1
References
Wu SH, Shu CM. Reactive hazard analysis of cumene hydroperoxide and dicumyl hydroperoxide. Process Saf Prog. 2009;29(2):162–5.
Wu SH. Process safety application and loss prevention of cumene hydroperoxide, Ph.D. Dissertation (2010), NYUST, Yunlin, Douliou, Taiwan, ROC
Luo KM, Chang JG, Lin SH, Chang CT, Yeh TF, Hu KH, Kao CS. The criterion of critical runaway and stable temperatures in cumene hydroperoxide reaction. J Loss Prev Process Ind. 2011;14:229–39.
Peng JJ, Wu SH, Hou HY, Lin CP, Shu CM. Thermal hazards evaluation of cumene hydroperoxide mixed with its derivatives. J Therm Anal Calorim. 2009;96:783–7.
Shen SJ, Wu SH, Chi JH, Wang YW, Shu CM. Thermal explosion simulation and incompatible reaction of dicumyl peroxide by calorimetric technique. J Therm Anal Calorim. 2010;102:569–77.
Chen KY, Wu SH, Wang YW, Shu CM. Runaway reaction and thermal hazards simulation of cumene hydroperoxide by DSC. J Loss Prev Process Ind. 2008;21:101–9.
Huang CC, Peng JJ, Wu SH, Hou HY, You ML, Chu CM. Effects of cumene hydroperoxide on phenol and acetone manufacturing by DSC and VSP2. J Therm Anal Calorim. 2010;102:579–85.
Wu SH, Wang YW, Wu TC, Hu WN, Shu CM. Evaluation of thermal hazards for dicumyl peroxide by DSC and VSP2. J Therm Anal Calorim. 2008;93(1):189–94.
Chi JH, Wu SH, Charpentier JC, I YP, Shu CM. Thermal hazard accident investigation of hydrogen peroxide mixing with propanone employing calorimetric approaches. J Loss Prev Process Ind. 2012;25:142–7.
Chou HC, Wu SH, Chiang CC, Horng JJ, Chi JH, Shu CM. Effects of stirring rate for thermal runaway reaction in cumene hydroperoxide manufacturing process using calorimetric techniques. J Therm Anal Calorim. 2011;106:243–8.
Wu SH, Chou HC, Pan RN, Huang YH, Horng JJ, Chi JH, Shu CM. Thermal hazard analyses of organic peroxides and inorganic peroxides by calorimetric approaches. J Therm Anal Cal. 2012. doi:10.1007/s10973-011-1749-5
Wu SH. Thermal hazard investigation of cumene hydroperoxide in the first oxidation tower. J Therm Anal Cal. 2012; doi:10.1007/s10973-011-1821-1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, SH., Wu, JY., Wu, YT. et al. Explosion evaluation and safety storage analyses of cumene hydroperoxide using various calorimeters. J Therm Anal Calorim 111, 669–675 (2013). https://doi.org/10.1007/s10973-012-2570-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2570-5