Abstract
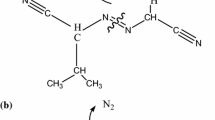
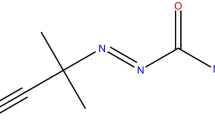
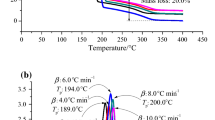
Azo compounds (azos) are radical polymerization initiators due to an azo group in the molecule. The molecular structure of the bivalent–N–N–composition is a weak bond which can release a significant amount of heat and toxic gas via thermal decomposition under high ambient temperature. This sequence on sensibility study aimed at the thermal hazard evaluation for the reactive and incompatible characteristics of azo compounds mixed with acid or alkaline solutions. To realize the thermal stability of azobis dimethylvaleronitrile (ABVN) during the process, transportation, and storage, we used differential scanning calorimetry and thermal activity monitor III to analyze the inherent safety properties and potential hazards. Isothermal and non-isothermal scanning tests were performed to compare the exothermic behaviors in a thermal decomposition process. Moreover, the thermal reactivity properties obtained via vent sizing package 2 were applied for evaluation, and the effects of thermal runaway reactions hazard were compared for ABVN with incompatibilities under the adiabatic conditions. These results are critically important in reactor design for producing and using azos to improve feeding safety, especially under thermal upsets.










Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor
- (dT dt −1)max :
-
Maximum self-heating rate (°C min−1)
- (dP dt −1)max :
-
Maximum self-pressure rise rate (psig min−1)
- E a :
-
Apparent activation energy (kJ mol−1)
- ∆H d :
-
Heat of decomposition (J g−1)
- ∆H f :
-
Heat of fusion (J g−1)
- k :
-
Reaction rate constant (min−1)
- k isoi :
-
Reaction rate constant under isothermal condition (min−1, i = 1, 2)
- P max :
-
Maximum pressure (psig)
- Q max :
-
Maximum peak power at time (W g−1)
- R :
-
Ideal gas law constant (8.31415 J K−1 mol−1)
- T 0 :
-
Onset temperature (°C)
- T :
-
Absolute temperature (K)
- T p :
-
Peak temperature (°C)
- T max :
-
Maximum temperature (°C)
- T m :
-
Melting temperature (°C)
- TMR iso :
-
Time to maximum rate under isothermal conditions (h)
- TMR ad :
-
Time to maximum rate under adiabatic condition (h)
- β:
-
Heating rate (°C min−1)
- T NR :
-
Temperature of no return (°C)
- W p :
-
Peak power (W g−1)
- α:
-
Conversion rate (%)
- R 2 :
-
Correlation coefficient (dimensionless)
- Ø:
-
Thermal inertia (dimensionless)
References
Classification and Labelling of Chemicals Global Harmonised System (GHS), Council of Labor Affairs, Taiwan, ROC. 2005.
Chen CH. Thermal runaway hazard studies for azobisisobutyronitrile with incompatible substances. National Yunlin University of Science & Technology, Master Thesis, Yunlin, Taiwan, ROC. 2012.
Li XR, Wang XL, Koseki H. Study on thermal decomposition characteristics of AIBN. J Hazard Mater. 2008;159:13–8.
The United Nations, Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria of UN, 18th ed., New York and Geneva, 2013, p. 179–371.
Liu SH, Yu YP, Lin YC, Weng SY, Hsieh TF, Hou HY. Complex thermal evaluation for 2,2′-azobis (isobutyronitrile) by non-isothermal and isothermal kinetic analysis methods. J Therm Anal Calorim. 2014;116:1361–7.
Grewer T, Frurip DJ, Harrison BK. Prediction of thermal hazards of chemical reactions. J Loss Prev Process Ind. 1999;12:391–8.
Chi JH, Wu SH, Shu CM. Thermal explosion analysis of methyl ethyl ketone peroxide by non-isothermal. J Hazard Mater. 2009;171:1145–9.
Zhang T, Xie CX, Jin MP, Sun F, Zhang LL. Study on thermal hazard of ABVN and Influence of Impurities. Acta Physico Chimica Sinica. 2012;122–7.
Wei W. Study on thermal hazards of oil-soluble azo initiators. Nanjing University of Science & Technology, Master Thesis, Nanjing, PR China. 2013.
Li XR, Koseki H. Thermal decomposition kinetic of liquid organic peroxides. J Loss Prev Process Ind. 2005;18:460–4.
Zhang T, Xie CX, Jin MP, Sun F, Zhang LI. Study on thermal hazard of ABVN and influence of impurities. C S S J. 2012;22:122–7.
Li XR, Koseki H. Study on the early stage of runaway reaction using Dewar vessels. J Loss Prev Process Ind. 2005;18:455–9.
Liu SH, Hou, HY, Chen JW, Weng SY, Lin YC, Shu CM. Effects of thermal runaway hazard for three organic peroxides conducted by acids or alkalines with DSC, VSP2, and TAM III. Thermochim Acta. 2013;226–32.
Whitmore MW, Wilberforce JK. Use of the accelerating rate calorimeter and the thermal activity monitor to estimate stability temperatures. J Loss Prev Process Ind. 1993;6:95–102.
Wei C, Saraf SR, Rogers WJ, Mannan MS. Thermal runaway reaction hazards and mechanisms of hydroxylamine with acid/base contaminants. Thermochim Acta. 2004;421:1–9.
Li XR, Koseki H. Study on the early stage of runaway reaction under adiabatic conditions. J Loss Prev Process Ind. 2005;18:455–9.
Mettler Company, 821e operation instructions, 2014.
STARe Software with Solaris Operating System. Operating instructions. Switzerland: Mettler Toledo; 2004.
Product Information, TAM III Thermostat. www.thermometric.com. 2014.
Tseng JM, Shu CM. Isothermal kinetic evaluation of methyl ethyl ketone peroxide mixed with acetone by TAM III tests. Thermochim Acta. 2010;507–508:45–58.
VSP2 Manual and Methodology. Fauske & Associates Inc. Illinois: Burr Ridge; 2003.
Askonas CF, Burelbach JP, Leung JC. The versatile VSP2: A tool for adiabatic thermal analysis and vent sizing applications. In: North American Thermal Analysis Society, 28th Annual Conference, Orlando, FL, USA. 4–6 Oct 2000.
Wang YW, Duh YS, Shu CM. Evaluation of adiabatic runaway reaction and vent sizing for emergency relief from DSC calorimetry. Ind Eng Chem Res. 2001;40:1125–32.
Leung JC, Fauske HK, Fisher HG. Thermal runaway reactions in a low thermal inertia apparatus. Thermochim Acta. 1986;104:13–29.
Huang CC, Peng JJ, Wu SH, Hou HY, You ML, Shu CM. Effects of cumene hydroperoxide on phenol and acetone manufacturing by DSC and VSP2. J Therm Anal Calorim. 2010;102:578–85.
Dubikhin VV, Knerel’man EI, Manelis GB, Nazin GM, Prokudin VG, Stashina GA, Chukanov NV, Shastin AV. Thermal decomposition of azobis (isobutyronitrile) in the solid state. Kinet Catal. 2012;446:171–5.
Peng MJ, Lu GB, Chen WH, Chen LP, Lu JY. Thermal decomposition characteristic and kinetics of AIBN in aniline solvent. Acta Phys. 2013;29:2095–100.
Ng WL. Thermal decomposition in the solid state. Aust J Chem. 1975;28:1169–78.
Hou HY, Su CH, Shu CM. Thermal risk analysis of cumene hydroperoxide in the presence of alkaline catalysts. J Loss Prev Process Ind. 2012;25:176–80.
Ferguson HD, Townsend DI, Hofelich TC, Russell PM. Reactive chemicals hazard evaluation: impact of thermal characteristics of transportation/storage vessels. J Hazard Mater. 1994;37:285–302.
Semenov NN. Some problems of chemical kinetics and reactivity, vol. 2. New York: Pergamon Press; 1959.
Weng SY, Liu SH, Tsai LC, Hsieh TF, Ma CM, Shu CM. Thermokinetics simulation for multi-walled carbon nanotubes with sodium alginate by advanced kinetics and technology solutions. J Therm Anal Calorim. 2013;113:1603–10.
Lin CP, Tseng JM, Chang YM, Cheng YC, Lin HY, Chien CY. Green thermal analysis for predicting thermal hazard of storage and transportation safety for tert-butyl peroxybenzoate. J Loss Prev Process Ind. 2012;25:1–7.
Brauner N, Shacham M. Statistical analysis of linear and non-linear correlation of the Arrhenius equation constants. Chem Eng Process. 1997;36:243–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, SH., Chen, YC. & Hou, HY. Thermal runaway hazard studies for ABVN mixed with acids or alkalines by DSC, TAM III, and VSP2. J Therm Anal Calorim 122, 1107–1116 (2015). https://doi.org/10.1007/s10973-015-4789-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4789-4