Abstract
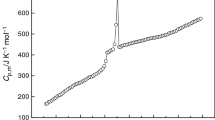
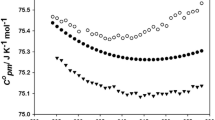
The ionic liquid 1-(2-methoxyethyl)-3-ethyl imidazolium perrhenate [C22O1IM][ReO4] was prepared in the laboratory firstly, and its structure was confirmed by 1H NMR and 13C NMR. The heat capacities were precisely measured in the temperature range from 78 to 392 K by means of a fully automated adiabatic calorimeter. For [C22O1IM][ReO4], the melting temperature, standard molar heat capacity, molar enthalpy, and molar entropy of solid–liquid phase transition were determined to be (211.826 ± 0.130) K, (142.31 ± 0.34) J K−1 mol−1, (14.382 ± 0.046) kJ mol−1, and (67.93 ± 0.22) J K−1 mol−1, respectively. And the experimental values of molar heat capacities were fitted to a polynomial equation using least square method in the appropriate temperature ranges. The thermodynamic functions (HT − H298.15) and (ST − S298.15) were also obtained from the heat capacity data in the experimental temperature range with an interval of 5 K.



Similar content being viewed by others
References
Tong B, Liu QS, Tan ZC, Urs WB. Thermochemistry of alkyl pyridinium bromide ionic liquids: calorimetric measurements and calculations. J Phys Chem A. 2010;114:3782–7.
Baudequin C, Bregeon D, Levillain J. Chiral ionic liquids, a renewal for the chemistry of chiral solvents? Design, synthesis and applications for chiral recognition and asymmetric synthesis. Tetrahedron Asymmetry. 2005;16:3921–45.
Clavier H, Boulanger L, Audi N, Toupet L, Mauduit M, Guillemin JC. Design and synthesis of imidazolinium salts derived from (l)-valine. Investigation of their potential in chiral molecular recognition. Chem Commun. 2004;10:1224–5.
Yoshitaka S, Yoko O, Yasuhisa Y, Kazuya S, Tooru A. Low-temperature heat capacity of room-temperature ionic liquid, 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J Phys Chem B. 2006;110:13970–5.
Paulechka YU, Kabo AG, Blokhin AV. Calorimetric determination of the enthalpy of 1-butyl-3-methylimidazolium bromide synthesis: a key quantity in thermodynamics of ionic liquids. J Phys Chem B. 2009;113:14742–6.
Fukumoto K, Yoshizawa M, Ohno H. Room temperature ionic liquids from 20 natural amino acids. J Am Chem Soc. 2005;127:2398–9.
Fang DW, Tong J, Guan W, Wang H, Yang JZ. Prediction of the thermodynamic properties of 1-alkyl-3-methylimidazolium lactate ionic liquids [Cnmim][Lact] (n = 2, 3, 4, 5, and 6) by parachor. Sci China Chem. 2010;53:2564–70.
Currás MR, Gomes MFC, Husson P, Padua AAH, Garcia J. Calorimetric and volumetric study on binary mixtures 2,2,2-trifluoroethanol + (1-butyl-3-methylimidazolium tetrafluoroborate or 1-ethyl-3-methylimidazolium tetrafluoroborate). J Chem Eng Data. 2010;55:5504–12.
Jiang YY, Wang GN, Zhou Z, Wu YT, Geng J, Zhang ZB. Tetraalkylammonium amino acids as functionalized ionic liquids of low viscosity. Chem Commun. 2008;4:505–7.
Lv XC, Tan ZC, Gao XH, Sun LX. Molar heat capacity and thermodynamic properties of Lu(C5H9NO4)(C3H4N2)6(ClO4)3 5HClO4 10H2O. J Therm Anal Calorim. 2013;113:971–6.
Tan ZC, Shi Q, Liu BP, Zhang HT. A fully automated adiabatic calorimeter for heat capacity measurement between 80 to 400 K. J Therm Anal Calorim. 2008;92:367–74.
Earle MJ, McCormac PB, Seddon KR. Diels–Alder reactions in ionic liquids. A safe recyclable alternative to lithium perchlorate–diethyl ether mixtures. Green Chem. 1999;1:23–5.
Pan Y, Zheng L, Xing NN, Ji HX. The molar surface Gibbs free energy and estimate of polarity for a new ether-functionalized ionic liquid [C22O1IM][DCA]. J Chem Thermodyn. 2017;112:213–9.
Dupont J, Suarez PAZ, Souza RF, Burrow RA, Kintzinger JP. C—H-π interactions in 1-n-Butyl-3-methylimidazolium tetraphenylborate molten salt: solid and solution structures. Chem Eur J. 2000;6:2377–81.
Liu QB, Janssen MHA, Rantwijk F, Sheldon RA. Room-temperature ionic liquids that dissolve carbohydrates in high concentrations. Green Chem. 2005;7:39–42.
Ditmars DA, Ishihara S, Chang SS, Bernstein G, Wes ED. Enthalpy and heat-capacity standard reference material: synthetic sapphire (α-Al2O3) from 10 to 2250 K. J Res Natl Bur Stand. 1982;87:159–63.
Fang DW, Zuo JT, Xia MC, Tong J, Li J. Low-temperature heat capacities and the thermodynamic functions of ionic liquids 1-heptyl-3-methyl imidazolium perrhenate. J Therm Anal Calorim. 2018;132:2003–8.
Tan ZC, Sun LX, Meng SH, Li L, Zhang JB. Heat capacities and thermodynamic functions of p-chlorobenzoic acid. Chem J Chin Univ. 2002;34:1417 (in Chinese).
Tan ZC, Sun GY, Song YJ, Wang L, Han JR, Wang M. An adiabatic calorimeter for heat capacity measurement of small samples-the heat capacity of nonlinear optical materials KTiOPO4 and RbTiOAsO4 crystals. Thermochim Acta. 2000;247:252–3.
Tan ZC, Di YY. Review of modern low-temperature adiabatic calorimetry. Prog Chem. 2006;18:1234 (in Chinese).
Acknowledgements
This project was financially supported by National Nature Science Foundation of China NSFC (Nos. 21673107 and 21703090) and Liaoning BaiQianWan Talents Program (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fang, DW., Gong, L., Fan, XT. et al. Low-temperature heat capacity and standard thermodynamic functions of the novel ionic liquid 1-(2-methoxyethyl)-3-ethyl imidazolium perrhenate. J Therm Anal Calorim 138, 1437–1442 (2019). https://doi.org/10.1007/s10973-019-08295-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08295-5