Abstract
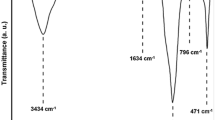
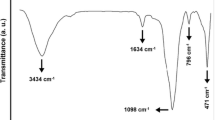
Mesoporous diatomite platelets were used for in situ copolymerization of styrene and butyl acrylate by activators generated by electron transfer for atom transfer radical polymerization (AGET ATRP) to synthesize tailor-made poly(styrene-co-butyl acrylate) nanocomposites. FTIR spectroscopy, nitrogen adsorption/desorption isotherm and thermo-gravimetric analysis (TGA) were employed for evaluating some inherent properties of the pristine diatomite platelets. Evaluation of pore size distribution and morphological studies were also performed by SEM and TEM. Conversion and molecular weight determinations were carried out using gas and size exclusion chromatography, respectively. Addition of 3 mass% pristine mesoporous diatomite leads to increase in conversion from 69 to 83%. Molecular weight of poly(styrene-co-butyl acrylate) chains increases from 7920 to 9940 g mol−1 by addition of 3 mass% pristine mesoporous diatomite; however, Đ values increase from 1.17 to 1.45. Appropriate agreement between theoretical and experimental molecular weight in combination with low Đ values can appropriately demonstrate the living nature of copolymerization. Copolymers composition was evaluated using 1H NMR spectroscopy. Increasing thermal stability of the nanocomposites is demonstrated by TGA. Differential scanning calorimetry shows an increase in glass transition temperature from 33 to 36 °C by adding 3 mass% of mesoporous diatomite platelets.








Similar content being viewed by others
References
Rao Y, Pochan JM. Mechanics of polymer–clay nanocomposites. Macromolecules. 2007;40:290–6.
Kumara A, Ghosha PK, Yadavb KL, Kumar K. Thermo-mechanical and anti-corrosive properties of MWCNT/epoxy nanocomposite fabricated by innovative dispersion technique. Compos Part B Eng. 2017;113:291.
Janowska G, Mikołajczyk T, Olejnik M. Thermal properties and flammability of fibres made from polyimidoamide nanocomposite. J Therm Anal Calorim. 2007;88:843–9.
Khezri K, Mahdavi H. Polystyrene-silica aerogel nanocomposites by in situ simultaneous reverse and normal initiation technique for ATRP. Microporous Mesoporous Mater. 2016;228:132–40.
Nguyen Q, Baird D. Preparation of polymer–clay nanocomposites and their properties. Adv Polym Technol. 2006;25:270–85.
Paul DR, Robeson LM. Polymer nanotechnology: nanocomposites. Polymer. 2008;49:3187–204.
Fazli Y, Khezri K. Mesoporous diatomite-filled PMMA by in situ reverse atom transfer radical polymerization. Colloid Polym Sci. 2017;295:247.
Roghani-Mamaqani H, Khezri K. Polystyrene-attached graphene nanolayers by reversible addition-fragmentation chain transfer polymerization: a grafting from epoxy groups with various densities. J Polym Res. 2016;23:190–204.
Nenadovic S, Nenadovic M, Kovacevic R, Matovic L, Matovic B, Jovanovic Z, Grbovic Novakovic J. Influence of diatomite microstructure on its adsorption capacity for Pb(II). Sci Sinter. 2009;41:309–17.
Jung K, Jang D, Ahn K. A novel approach for improvement of purity and porosity in diatomite (diatomaceous earth) by applying an electric field. Int J Miner Process. 2014;131:7–11.
Jian Z, Qingwei P, Meihong N, Haiqiang S, Na L. Kinetics and equilibrium studies from the methylene blue adsorption on diatomite treated with sodium hydroxide. Appl Clay Sci. 2013;83–84:12–6.
Al-Ghouti MA, Khraisheh MAM, Ahmad MN, Allen SJ. Microcolumn studies of dye adsorption onto manganese oxides modified diatomite. J Hazard Mater. 2007;146:316–27.
Guo S, Shi L. Synthesis of succinic anhydride from maleic anhydride on Ni/diatomite catalysts. Catal Today. 2013;212:137–41.
Caliskan N, Kul AR, Alkan S, Sogut EG, Alacabey I. Adsorption of Zinc(II) on diatomite and manganese-oxide-modified diatomite: a kinetic and equilibrium study. J Hazard Mater. 2011;193:27–36.
Sheng G, Dong H, Li Y. Characterization of diatomite and its application for the retention of radiocobalt: role of environmental parameters. J Environ Radioact. 2012;113:108–15.
Yuan P, Liu D, Tan D, Liu K, Yu H, Zhong Y, Yuan A, Yu W, He H. Surface silylation of mesoporous/macroporous diatomite (diatomaceous earth) and its function in Cu(II) adsorption: the effects of heating pretreatment. Microporous Mesoporous Mater. 2013;170:9–19.
Jiang L, Liu L, Xiao S, Chen J. Preparation of a novel manganese oxide-modified diatomite and its aniline removal mechanism from solution. Chem Eng J. 2016;284:609.
Zetterlund PB, Kagawa Y, Okubo M. Controlled/living radical polymerization in dispersed systems. Chem Rev. 2008;108:3747–94.
Sarsabili M, Kalantari K, Khezri K. SR&NI atom transfer radical random copolymerization of styrene and butyl acrylate in the presence of MPS-functionalized silica aerogel nanoparticles. J Therm Anal Calorim. 2016;126:1261–72.
Khezri K, Fazli Y. Synthesis and characterization of poly (styrene-co-butyl acrylate)/silica aerogel nanocomposites by in situ AGET ATRP: investigating thermal properties. High Temp Mater Process. 2016. doi:10.1515/htmp-2015-0244.
Kagawa Y, Zetterlund PB, Minami H, Okubo M. Compartmentalization in atom transfer radical polymerization (ATRP) in dispersed systems. Macromol Theory Simul. 2006;15:608–13.
Roghani-Mamaqani H, Khezri K. A grafting from approach to graft polystyrene chains at the surface ofgraphene nanolayers by RAFT polymerization: various graft densities from hydroxyl groups. Appl Surf Sci. 2016;360:373–82.
Chmielarz P. Synthesis of multiarm star block copolymers via simplified electrochemically mediated ATRP. Chem Paper. 2017;71:161.
Sarsabili M, Rahmatolahzadeh R, Shobeiri SA, Hamadanian M, Farazin A, Khezri K. Reverse atom transfer radical random copolymerization of styrene and methyl methacrylate in the presence of diatomite nanoplatelets. Polym Adv Technol. 2017. doi:10.1002/pat.4131.
Karaman S, Karaipekli A, Sarı A, Bicer A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Solar Energy Mater Solar Cells. 2011;95:1647–53.
Li X, Bian C, Chen W, He J, Wang Z, Xu N, Xue G. Polyaniline on surface modification of diatomite: a novel way to obtain conducting diatomite fillers. Appl Surf Sci. 2003;207:378–83.
Li X, Li X, Wang G. Fibrillar polyaniline/diatomite composite synthesized by one-step in situ polymerization method. Appl Surf Sci. 2005;249:266–70.
Hu S, Zhu X, Hu W, Yan L, Cai C. Crystallization behaviors and foaming properties of diatomite-filled polypropylene composites. Polym Bull. 2013;70:517–33.
Liang JZ. Effects of extrusion conditions on die-swell behavior of polypropylene/diatomite composite melts. Polym Test. 2008;27:936–40.
Garderen N, Clemens FJ, Mezzomo M, Bergmann CP, Graule T. Investigation of clay content and sintering temperature on attrition resistance of highly porous diatomite based material. Appl Clay Sci. 2011;52:115–21.
Sun Z, Yang X, Zhang G, Zheng S, Frost RL. A novel method for purification of low grade diatomite powders in centrifugal fields. Int J Miner Process. 2013;125:18–26.
Liu D, Yuan P, Tan D, Liu H, Wang T, Fan M, Zhu J, He H. Facile preparation of hierarchically porous carbon using diatomite as both template and catalyst and methylene blue adsorption of carbon products. J Colloid Interface Sci. 2012;388:176–84.
Zhang L, Cheng Z, Lu Y, Zhu X. A highly active iron-based catalyst system for the AGET ATRP of styrene. Macromol Rapid Commun. 2009;30:543–7.
Davoudizadeh S, Sarsabili M, Khezri K. Synthesis and characterization of polystyrene/mesoporous diatomite composites via activators generated by electron transfer for atom transfer radical polymerization. Z Phys Chem. 2017;231:1543.
Wu D, Yang Y, Cheng X, Liu L, Tian J, Zhao H. Mixed molecular brushes with PLLA and PS side chains prepared by AGET ATRP and ring-opening polymerization. Macromolecules. 2006;39:7513–9.
Asfadeh A, Haddadi-Asl V, Salami-Kalajahi M, Sarsabili M, Roghani-Mamaqani H. Investigating the effect of MCM-41 nanoparticles on the kinetics of atom transfer radical polymerization of styrene. NANO. 2013;8:1350018.
Khezri K, Fazli Y. Characterization of diatomite platelets and its application for in situ atom transfer radical random copolymerization of styrene and butyl acrylate: normal approach. J Inorg Organomet Polym. 2017;27:266–74.
Khezri K, Fazli Y. Polystyrene/mesoporous diatomite composites by in situ simultaneous reverse and normal initiation technique for atom transfer radical polymerization. Polym Sci Ser B. 2017;59:109–16.
Khezri K, Najafi M, Roghani-Mamaqani H. Reversible addition fragmentation chain transfer polymerization of styrene from the edge of graphene oxide nanolayers. J Polym Res. 2017;24:34–46.
Khezri K, Mahdavi H. Polystyrene–silica aerogel nanocomposites by in situ simultaneous reverse and normal initiation technique for ATRP. Microporous Mesoporous Mater. 2016;228:132.
Khezri K. Polystyrene–mesoporous diatomite composites produced by in situ activators regenerated by electron transfer atom transfer radical polymerization. RSC Adv. 2016;6:109286–95.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davoudizadeh, S., Ghasemi, M., Khezri, K. et al. Poly(styrene-co-butyl acrylate)/mesoporous diatomaceous earth mineral nanocomposites by in situ AGET ATRP. J Therm Anal Calorim 131, 2513–2521 (2018). https://doi.org/10.1007/s10973-017-6771-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6771-9