Abstract
The phenolic urethane cold-box (PUCB) process was first introduced to the foundry industry in the late 1960s. Since then, it has become one of the most popular methods to make foundry purpose sand moulds and cores, utilized in the manufacturing of aluminium and cast iron cast components. The factors to be considered, affecting the general performance of a PUCB moulding mixture, are the temperature of sand, the moisture content, the mixing conditions, etc. Moreover, there are variable production parameters such as binder level, to improve certain properties of the mould and/or the core based on their specific area of application. These are mainly mechanical properties such as tensile or splitting strength. They have significant influences on the behaviour of the moulding material and are usually tested at room temperature. Although the production phases of the PUCB system are refined to a high extent today, the effect of binder content on the quality of the mould/core and the final casting should be supported by new approaches also in thermal sciences, interpreted in high-temperature environment. In this work, different PUCB mixtures were produced to evaluate the effect of various binder levels on the thermophysical properties of sand cores. Thermogravimetry, differential thermal analysis and a novel application of Fourier thermal analysis were used to study the decomposition processes of the PUCB mixture and to reveal the impact of binder level on the heat absorption (cooling) capacity of sand cores at temperatures relevant in the manufacturing of cast iron parts (1300 ± 10 °C).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “cold-box” implies the room temperature curing of the sand-binder mixture accelerated by vapour or gas catalyst that is passed through the sand. The phenolic urethane cold-box (PUCB) process is a three-part system, with two liquid binder components. Part I is the reactive component, a phenol–formaldehyde polymer blended with solvents and additives to produce a low-viscosity resin solution. Part II is polymeric isocyanate (polyisocyanate), again blended with solvents and additives. The hydroxyl groups provided by Part I react with the isocyanate groups in Part II forming a solid urethane polymer (polyurethane, PUR) in the presence of the third component, a gaseous catalyst. Two types of tertiary amine catalysts are commonly used to cure the resin and the isocyanate: triethylamine (TEA) and dimethylethylamine (DMEA). A simplified version of the curing mechanism is:
The urethane reaction does not produce water or any other by-product. The system contains 3–4% nitrogen, which comes from the polymeric isocyanate component. The PUCB system can be applied with most types of sands commonly used for mould and core making in the foundry industry. Some consideration must be given, however, to the effects of sand temperature, chemistry and moisture content on the bonding performance. The ideal sand temperature is 20–25 °C, and the maximum tolerance in sand moisture content is 0.2 mass% [1, 2].
Various sand-mixing equipments can be used with the process. By the PUCB system, the sand mixture is usually blown into a pattern (core box) by compressed air. In core blowing operations, low pressures of 200–300 kPa are general. In most cases, 1–3 mass% total binder level consisting of Part I and Part II in 50:50 ratio is mixed to the clean sand. Various designs of generators vapourize and blend the amines with carrier gas (air or CO2) for the gassing. Approximately 1 mL of liquid amine is needed per kilogram of sand. Typical gassing times are 1–2 s followed by a longer period of air purge (10–15 s) to remove residual amine from the core [1,2,3,4,5].
The exhaust gas from the core box during the gassing and purging cycle will contain a certain amount of amine catalyst. This exhaust gas is recommended to be collected in a chemical scrubber for safety reasons. The scrubber passes the exhaust gas through a solution of dilute acid and neutralizes the amine to form nonvolatile acid salt. Another environmental issue is the high flammability of liquid amine; moreover, the air/amine mixture may be explosive. TEA and DMEA act as a respiratory irritant and may cause asthmatic symptoms. Like in other organic resin/binder applications in the foundries, formation of hazardous air pollutants and emission of organic compounds due to the decomposition reactions must be also considered to ensure an environmentally sustainable and safe working area in the foundry. Besides the known environmental and safety drawbacks, the major advantages of the PUCB process are the fast cure cycles and the excellent dimensional accuracy of cores and moulds [5,6,7].
Materials
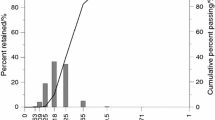
Three PUCB mixtures with different binder levels were studied. All three consisted of foundry purpose silica sand as refractory, foundry grade phenolic resin, polymeric isocyanate and amine vapour as catalyst. The applied silica sand was light brown coloured and sub-rounded shaped with a medium grain size of 0.28 mm. Grain size distribution is shown in Fig. 1. Specific surface area was 142 cm2 g−1.
The phenolic resin (Part I) consisted of three main components: formaldehyde, phenol and methanol. The resin and the 4.4′-diphenylmethane diisocyanate (MDI, isocyanate monomer) (Part II) were mixed together with the silica sand by an industrial foundry sand mixer. After compaction by blowing, the mixtures were gas-cured by triethylamine (TEA) vapour for 1 s and purged by hot air for 7 s. Table 1 shows the production parameters of the PUCB mixtures studied.
To avoid deviations of the thermal behaviour due to different initial parameters like density, free moisture and loss on ignition (LOI), sample preparation and storage conditions were maintained precisely. Samples taken from each mixture were dried at 105 °C for one hour to monitor the free moisture content. LOI values were determined in the dried specimens at 900 °C for 90 min (Table 2).
Mixture I was studied by TG–DTA to get an insight about the decomposition process of the PUCB mixture in general. TG–DTA was performed on a MOM Budapest derivatograph C/PC under static air atmosphere. The heating rate was set to 10 °C min−1; the reference material was α-Al2O3. Samples of 300 mg were placed in ceramic crucibles.
Figure 2 shows the results of the TG–DTA. The multi-staged decomposition of the PUCB mixture started with the vapourization of free moisture at 100 °C. The process continued with the degradation of bound water and solvents in the binder components. Endotherm processes appeared on the DTA curve at temperatures 250 °C (A) and from 350 to 450 °C (B) representing the degradation of the polyurethane. At the same time, static air atmosphere allowed the decomposition products to combust, according to the exotherm peak at around 500 °C (C). The TG curve showed that the binder burned out completely up to approximately 600 °C. The total mass loss of ~1.25% corresponds well with the moisture content and LOI result of Mixture I. Heat absorbed by the allotropic transformation of silica sand from α-quartz to β-quartz also appears on the DTA curve (small endotherm peak D at 573 °C). The minor mass loss and endotherm peak at approximately 750 °C are assumed to be related to the degradation/transition of impurities in the foundry purpose silica sand.
Experimental
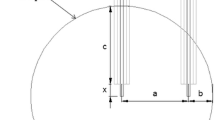
Spherical sand samples made of the three PUCB mixtures were prepared with different diameters of 40, 50 and 60 mm to run Fourier thermal analysis. N-type mineral-insulated thermocouples with stainless steel sheath were used for temperature measurements in the samples, one measuring point was in the geometrical centre of the cores, and another lateral measuring point was near the sample wall (Fig. 3). Positions of temperature measuring points concerning all three sample diameters were similar to the dimensions applied in previous works (Table 3) [8, 9]. Samples were immersed into liquid cast iron (1300 ± 10 °C) to study the temperature distribution in the cores. Quartz glass pipes with 1-mm wall thickness (t1 and t2) and various dimensions were used to avoid the direct contact of thermocouples with the melt during the tests (Table 4). Neither preliminary drying nor coating of the specimens were applied.
Heat distribution in the PUCB mixtures
Figure 4 shows the temperature distribution versus time in the 50-mm-diameter samples of all three PUCB mixtures. Temperature recordings in the lateral points near the sample walls showed faster heating (Fig. 4b), compared to the curves in the central measuring point (Fig. 4a). The difference in heating rates based on the positions of the temperature recordings is also obvious on the curves of 40- and 60-mm sample diameters (Figs. 8, 9 in Appendix 1). However, differences due to the various binder levels in the mixtures did not appear clearly. The reason for this is the temperature of the liquid cast iron, which ensures a generally high heating rate and a relatively fast procession of the expected heat-absorbing and binder degradation processes. Thus, variations of heating rates by different sample diameters did not enhance the clear visibility of these processes either (Fig. 5).
Results of Fourier thermal analysis
Recording and interpretation of temperature variation in time of a cooled or heated material is widely used today in foundry technology [10]. The broad areas of application cover works based on cooling curve analysis of both nonferrous and ferrous alloys [11,12,13], and studies applying traditional methods such as TG-DSC-DTG to examine materials suitable for bonding agents in foundry purpose mould and core making technology [14, 15]. Other works deal with the measurement of thermophysical properties in greensand moulds applying real foundry conditions [16,17,18]. In this study, the method of Fourier thermal analysis (FTA) was applied in an inverse way to interpret the heat distribution versus time curves recorded in the spherical samples. The aim was to clarify the thermal aspects of the increasing binder levels in the PUCB mixtures, in addition to the known positive effects on the mechanical properties of the cores at room temperature. Thus, the total absorbed heat, the fraction of absorbed heat and the rate of heat absorption by the moulding mixture degradation were calculated to obtain novel information about the cooling capacity of the PUCB mixtures.
The heat transfer between the melt and the moulding material is the most important phenomenon in foundry technology, which ensures the necessary temperature gradient for the liquid to solid transformation to take place. The heat transfer is strongly affected by the ability of moulding materials to extract heat from the liquid metal. Moulds and cores with high heat absorption capacity can accelerate the cooling, while moulds and cores with low heat absorption capacity will decrease cooling rates. Thus, moulding materials with designed cooling capacity could modify the formation of the initial casting skin, which is a key moment in the early stages of the solidification, and in the formation of penetration, blow hole or even shrinkage-related casting defects. Nevertheless, to achieve the future scope of directional solidification by moulding mixture composition, the thermal effects of production parameters such as binder level carry both scientific and technological importance.
The calculated total absorbed heat values for all three binder levels are shown in Table 5. Closely equal results by each sample diameter are an evidence of the good reproducibility of the method. Total absorbed heat corresponds to the amount of heat taken away from the melt by the binder degradation processes and the phase transitions in the PUCB mixture. These are the vapourization of free moisture content, the degradation of the polyurethane binder between 150 and 600 °C and the transformation of silica sand from α-quartz to β-quartz at 573 °C.
Table 5 shows that 0.6 mass% additional binder level (0.3 mass% phenolic resin + 0.3 mass% isocyanate monomer) increased the total absorbed heat by approximately 20%. Therefore, this amount of binder addition will eventually improve the cooling capacity of the cores. Depending on the wall thickness of the casting, the above-mentioned effect is expected to shorten the total solidification time in the casting, to affect the microstructure and to result in certain variations in mechanical properties of the cast part.
The phenomenon was investigated and confirmed by earlier authors. They studied the effect of various mould materials on the cooling rate of cast iron castings [13]. They only distinguish metallic, sand, ceramic and insulated moulds. Based on their findings, the application of various mould materials may eventuate in more than 60 °C difference in the temperatures at the end of solidification. Other authors primarily focusing on sand moulds with different compositions concluded the significance of the type of sand on the final microstructure and the mechanical properties of aluminium alloys [19].
The effect of total binder levels on the cooling capacity can be further evaluated versus the temperature in the centres of the specimens. For this purpose, fraction of total absorbed heat and rate of heat absorption were also calculated.
Figure 6 shows the fraction of absorbed heat versus the temperature recorded in the centres of the 50-mm-diameter cores. The total amount of heat taken away from the melt was distributed evenly in the temperature range between 100 and 600 °C. Approximately 50% of the total heat was absorbed until the halfway of the heating process (at 300–350 °C). On the other hand, the multi-step nature of polyurethane degradation became more and more apparent as the total binder level increased, with the curve assigned to Mixture III (total binder level of 2.4%) showing the clearest changes in the decomposition characteristics. This feature was also apparent on the curves of 60-mm samples (Fig. 10b in Appendix 2), but less apparent on the curves of 40-mm sample diameters (Fig. 10a in Appendix 2). The reason for this is assumed to be the high heating rates in the smallest sample diameter. Analogously to the results of TG–DTA, the thermal decomposition of polyurethane was completed until 600 °C.
The rate of heat absorption versus the temperature recorded in the centres of the 50-mm-diameter cores showed better details about the polyurethane degradation (Fig. 7), representing the degradation characteristics of each mixture variables, this time in terms of cooling capacity versus temperature. The rate of heat absorption reached its first maximum (1) shortly after 100 °C, when the decomposition of bound water and solvents in the binder components occurred. The major stages of polyurethane degradation at 250 °C (2) and 350–450 °C (3) (identified also during the TG–DTA) also appeared clearly as notable maximums of heat absorption. Results showed that the increasing total binder level in the PUCB mixtures increased the maximum rates of heat absorption at the specific temperatures of the major degradation processes (1–3). This means that the cooling capacity of the PUCB mixtures was affected by the total binder level in the temperature interval from 100 to 400 °C in the core, e.g. starting from the very early stages of solidification. However, this phenomenon strongly depends on the variations in heating rates due to different sample diameters. Figure 11a in Appendix 2 shows that higher heating rates in the 40-mm samples caused even bigger differences in the maximums of heat absorption versus total binder level, compared to Fig. 7. Contrarily, the total binder level did not affect the maximums of cooling capacity in case of low heating rates provided by the largest cores (Fig. 11b in Appendix 2). This means that the effect of total binder level on the cooling capacity of thinner cores will be much more significant, compared to thicker cores.
Concluding remarks
In this work, the effect of variations in total binder level on the heat absorption capacity of PUCB mixtures was studied. In the first step, the thermal behaviour of the PUCB system was briefly discussed based on TG–DTA. Tests under constant heating rate of 10 °C min−1 and static air atmosphere presented valuable results; however, the mixtures were further studied by Fourier thermal analysis to obtain results demonstrating real foundry conditions. These were primarily ensured by the application of core wall thicknesses (40–60 mm) and heating rates (immersion in cast iron melt) prevalent in foundry technology.
The calculated total absorbed heat values showed that 0.6 mass% addition of total binder content (0.3 mass% phenolic resin + 0.3 mass% isocyanate monomer) to the PUCB mixture increased the total absorbed heat with approximately 20%. This feature is expected to improve the cooling capacity of the cores and to shorten the total solidification time in the casting. Results corresponded well with the conclusions drawn by earlier authors.
The heat absorption characteristics were further evaluated by the calculation of the fraction of absorbed heat and rate of heat absorption. Therefore, the effect of total binder level on the cooling capacity was evaluated also versus the temperature inside the specimens.
The fraction of absorbed heat curves indicated that the total amount of heat by binder degradation was distributed evenly in the temperature range of PUCB mixture degradation. The multi-step nature of the polyurethane decomposition identified during the TG–DTA was also apparent on the fraction of absorbed heat versus temperature curves in case of high binder levels and low heating rates.
The rate of heat absorption curves showed the degradation profile of the studied mixtures in terms of cooling capacity versus temperature. The increasing total binder level in the PUCB mixtures increased the maximum rates of heat absorption at the specific temperatures of the major polyurethane degradation processes. The cooling capacity of the cores was affected by the total binder level in the temperature interval from 100 to 400 °C in the core, e.g. starting from the very early stages of solidification. However, this phenomenon strongly depended on the heating rates provided by different sample diameters.
The results showed that the variation of total binder level in the PUCB system can modify the formation of the initial casting skin, which is a key moment in the early stages of the solidification and in the formation of penetration, blow hole or even shrinkage-related casting defects. Therefore, the solidification of the liquid metal could be controlled by the majority of the internal core solutions applied in foundry technology (≤50 mm), by the variations of the total binder levels in the PUCB mixture.
References
ASM metals handbook volume 15: castings. 4th edition. OH: ASM International, Metals Park; 1998. p. 212–21.
Chemically bonded cores & molds. Schaumburg, IL: American Foundry Society; 1987. p. 107–12.
Blackburn PA, Harry CM. A more productive phenolic urethane cold box process. Trans Am Foundry Soc. 1988;96:945–9.
Carey PR, Sturtz G. Sand binder systems part IV—urethane binders. Foundry Manag Technol. 1995;123(5):25–9.
Foseco Ferrous Foundryman’s handbook. Foseco International Ltd.; 2000. ISBN: 0 7506 4284 X.
Zhang H, Zhao H, Zheng K, Li X, Liu G, Wang Y. Diminishing hazardous air pollutant emissions from pyrolysis of furan no-bake binders using methanesulfonic acid as the binder catalyst. J Therm Anal Calorim. 2014;116:373–81.
Tiedje N, Crepaz R, Eggert T, Bey N. Emission of organic compounds from mould and core binders used for casting iron, aluminium and bronze in sand moulds. J Environ Sci Health Part A Tox Hazard Subst Environ Eng. 2010;45(14):1866–76.
Svidró JT, Diószegi A, Tóth J. The novel application of Fourier thermal analysis in foundry technologies. J Therm Anal Calorim. 2014;115:331–8.
Tóth J, Svidró JT, Diószegi A, Stevenson D. Heat absorption and binder degradation characteristics of 3D printed cores investigated by inverse thermal analysis. Int J Metalcast. 2016;10(3):306–14.
Stefanescu DM. Thermal analysis—theory and applications in metalcasting, the novel application of Fourier thermal analysis in foundry technologies. Int J Metalcast. 2015;9(1):7–22.
Krupinski M, Krupinska B, Rdzawski Z, Labisz K, Tanski T. Additives and thermal treatment influence on nonferrous alloys. J Therm Anal Calorim. 2015;120:1573–83.
Diószegi A, Diaconu VL, Fourlakidis V. Prediction of volume fraction of primary austenite at solidification of lamellar graphite cast iron using thermal analyses. J Therm Anal Calorim. 2016;124:215–25.
Stan S, Chisamera M, Riposan I, Barstow M. Application of thermal analysis to monitor the quality of hypoeutectic cast irons during solidification in sand and metal moulds. J Therm Anal Calorim. 2012;110:1185–92.
Zhang Y, Liu Q, Zeguang W, Zhang Y. Thermal behaviour analysis of two bentonite samples selected from China. J Therm Anal Calorim. 2015;121:1287–95.
Grabowska B, Malinowski P, Szucki M, Byczynski L. Thermal analysis in foundry technology. J Therm Anal Calorim. 2016;126:245–50.
Krajewski P K, Piwowarski G. Range of thermal conductivity changes of wet green foundry sand during casting solidification. Arch Metall Mater. 2015;60(3B):2391–5.
Krajewski P K, Piwowarski G, Zak P L, Krajewski W K. Experiment and numerical modelling the time of plate-shape casting solidification vs. thermal conductivity of mould material. Arch Metall Mater. 2014;59(4):1405–8.
Krajewski P K, Zovko-Brodarac Z, Krajewski W K. Heat exchange in the system mould-riser-ambient. Pt. 1, heat exchange coefficient from mould external surface. Arch Metall Mater. 2013;58(3):833–5.
Guanglei L, Naichao S, Shaochun S, Qinfang W. Effects of different casting mould cooling rates on microstructure and properties of sand-cast Al–7.5Si–4Cu alloy. China Foundry. 2013;10(6):396–400.
Acknowledgements
The present work was financed by the Swedish Knowledge Foundation. Cooperating parties in the project were Jönköping University, Scania CV AB and Volvo Powertrain Production Gjuteriet AB. External contribution was provided by the University of Miskolc. Participating persons from these institutions/companies are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Appendix 2
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Svidró, J.T., Diószegi, A., Svidró, J. et al. The effect of different binder levels on the heat absorption capacity of moulding mixtures made by the phenolic urethane cold-box process. J Therm Anal Calorim 130, 1769–1777 (2017). https://doi.org/10.1007/s10973-017-6611-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6611-y