Abstract
The most important advantage of foundry purpose moulding sand is that it can be reclaimed and reused through the casting manufacturing process. Supplying the foundry with a new source of material, sand reclamation brings along both environmental and economic advantages. Utilization of used sand can be considered as a common technological routine in the production of most types of chemically bound moulding materials. The epoxy-SO2 process is prevalent in the processing of cast iron engine components worldwide. Based on its excellent properties, it is mainly suitable for producing internal sand cores with complex geometry. Even though reclaimed sand addition is an active and well-functioning feature in ferrous foundries, the scientific and thermophysical background of its effects on the casting process is yet to be explored. In this work, the thermal aspects of different reclaimed sand levels in the epoxy-SO2 moulding system were examined. Thermogravimetry and differential thermal analysis of the epoxy-SO2 and reclaimed sand in focus were carried out to obtain basic understandings about their high-temperature behaviour. A state-of-the-art Fourier thermal analysis method presented in a recent paper was used at temperatures corresponding to actual cast iron production (1300 ± 10 °C), contrary to the previous tests at the typical temperature range of aluminium melt processing (660 ± 10 °C). By the right of the method, the effects of reclaimed sand addition on the heat absorption (cooling) capacity of the epoxy-SO2 moulding mixtures were investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The epoxy-SO2 process
The epoxy-SO2 process is an organic gas-cured method to produce sand cores, which means the hardening of the sand–organic resin mixture is accelerated by SO2 gas catalyst. The process utilizes a two-part liquid resin. Part I is a modified epoxy resin containing acrylic and epoxy functional components, and part II is cumene hydroperoxide as oxidizer. The mechanism that effectively cures the epoxy-acrylic resin is a combination of acid-induced and free radical-initiated polymerization reactions [1]. Besides foundry application, epoxy resins are also used as coatings, adhesives, laminates, semiconductor encapsulation, and matrices for advanced composites, based on their outstanding mechanical stiffness, toughness, chemical resistance, and superior adhesion [2].
Sand performance and casting properties are influenced by the ratio of acrylic and epoxy functional components. The resin is normally added in the range of 0.6–1.4 mass%, based on the mass of the sand and determined by the physical strength requirements of the core or the mould. The oxidizer is normally used between 30 and 50 mass% and calculated by the mass of the resin and the preferred curing rate. In high production, the compaction of the epoxy-SO2 system is achieved by blowing it into a pattern (core box) by compressed air in order to form the desired geometry [3, 4].
Specific applications may require preheated core boxes to improve cycle times. In typical core blowing operations, relatively low blowing pressures of 275–415 kPa are possible due to the excellent flow properties of the system. As components do not react with each other until the SO2 gas is introduced to the sand–resin mixture, the prepared material has an extremely long bench life compared to other cold-box and furan no-bake methods. This feature minimizes waste sand and decreases the machine downtime because sand containers and mixers do not have to be cleaned daily.
Once the sand is compacted, approximately one second of curing by SO2 catalyst is done with inert gas carriers such as nitrogen. It reacts with the cumene hydroperoxide and oxidizes into SO3 which forms sulphuric acid with the water in the system. This reaction provides the necessary acid media to obtain a cured polymer. A simplified version of the curing mechanism is:
Hot purging with air at 95 °C for around 10 s is recommended to achieve optimum cure and to remove residual gas from the core. Collection and neutralization of residual SO2 is necessary after the process from both safety and environmental reasons. In other cases, when the SO2 system is used with furan resin, the mixture can also release considerable aromatic hazardous air pollutants as they are thermally decomposed during the casting [3,4,5].
Scrubbing of the gas is usually done by a wet scrubbing unit that utilizes a shower of water and sodium hydroxide. The 5–10% solution of sodium hydroxide at a pH of 8–14 provides efficient neutralization of the SO2 and prevents the by-product (sodium sulphite) from precipitating out of the solution. Higher sodium hydroxide concentration will cause precipitation of the neutralized product [3, 4].
Sand reclamation
Reclamation is defined as the physical, chemical, or thermal treatment of a refractory aggregate to allow its reuse without significantly lowering its original advantageous properties as required for the application. To achieve this objective, one must evaluate the type of sand entering the reclamation system, the binder system used, and the area of reuse. The primary requirement is to remove the resin coating around the sand grains [3].
Before the sand is processed by a reclamation system, it must go through a preliminary preparation process. Sand lumps must be broken and ground to near individual grain size to expose the resin layer on individual grains to the process. Metallic and refuse such as wood and paper and other trash must be also removed. In some cases, cooling of the sand is necessary due to the relatively high temperature immediately after shakeout. Drums and fluid bed coolers are traditionally used to cool down the sand to adequate temperature [6].
There are three basic types of reclamation systems: wet, thermal, and dry. The selection depends greatly on the nature of resin/binder to be removed from the sand grains.
Wet hydraulic reclamation systems are used for clay (green sand) and silicate bonded mixtures. As these inorganic materials tend to melt rather than burn in a furnace, these sands are very difficult to reclaim by dry processes and are impossible to reclaim by thermal systems.
When the castings are all made in chemically bound sand moulds and cores, the sand can be reclaimed by thermal treatment. The gas- or oil-fired calcining ovens are operated at temperatures of 400–900 °C to promote the air oxidation of the residual binder amount. Following the calcining, the sand must be cooled down for reuse. Temperatures must be controlled carefully during thermal reclamation to avoid sintering reactions that cause the sand to agglomerate and stick to itself causing flowability problems.
Dry reclamation processes can be divided into pneumatic and mechanical scrubbing. The pneumatic system operates by impingement of a high velocity stream of air and sand grains. The process pulverizes the binder layers, and the dust debris is removed to a dust collector. Dust-containing residual must be collected as it is classified as dangerous waste.
In mechanical reclamation, the sand grains are hurdled at high velocity against a metallic barrier by an impeller causing sand-to-sand attrition. However, the residual bonding agents are not completely removed as some chemicals can be particularly tenacious in sticking to the sand grains. When this happens, it may be necessary to repeat the cycle several times. Mechanical reclamation units may be oriented either horizontally and vertically [5,6,7].
Materials
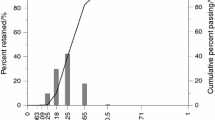
The “base” moulding mixture (without reclaimed sand addition) studied in this work consisted of washed and screened silica sand as basic refractory, fresh epoxy resin suitable for metal casting purposes and cumene hydroperoxide as oxidizing agent. The silica sand was light brown coloured and sub-rounded shaped with a medium grain size of 0.23 mm. Grain size distribution of the investigated sand is shown in Fig. 1. The measured specific surface area was 148 cm2 g−1, which is a significant parameter of a foundry aggregate when it comes to the resin demand necessary for adequate strength properties.
The epoxy resin (Part I) consisted of three main components: bisphenol A-epichlorohydrin resin, bisphenol F-epichlorohydrin resin, and trimethylolpropane triacrylate. It was mixed with the silica sand together with cumene hydroperoxide as oxidizing agent (Part II) by a conventional foundry sand mixer. Samples were then compacted by blowing and cured by SO2 gas. Composition and production parameters of the “base” moulding mixture are given in Table 1.
The properties of the “base” mixture and the storage conditions were maintained carefully. Discrepancy of the density, the free moisture content, and the loss on ignition (LOI) can significantly influence the thermophysical and decomposition behaviour. Samples taken from the “base” epoxy-SO2 system were dried at 105 °C for 1 h to measure the free moisture content. LOI values were then determined in the dried samples at 900 °C for 90 min (Table 2).
The “base” mixture was examined by TG–DTA to have initial information about the thermal profile of the moulding material in focus and to reveal its important decomposition features. TG–DTA was performed on a MOM Budapest derivatograph C/PC under static air atmosphere. The heating rate was initially set to 10 °C min−1, and the reference material was α-Al2O3. Samples of 300 mg were placed in ceramic crucibles.
Figure 2 shows the results of the TG–DTA. The epoxy resin started to decompose around 150 °C, when the free moisture was already vaporized. Minor endotherm peaks (A and B) on the DTA curve in the temperature interval between 200 and 550 °C show the complex endothermic process of the resin decomposition, which is overlapping with the combustion of the degradation products formed. Therefore, the real endothermic peaks can hardly be separated from the strongly drifting baseline of the DTA curve. Based on the transition of the TG curve at approximately 550 °C, the resin has burned out completely until this temperature. Total mass loss value of ~1.1% corresponded well to the free moisture content and LOI results. Allotropic transformation of silica sand from α-quartz to β-quartz also appeared on the DTA curve as an endotherm peak (C) at 573 °C.
The reclaimed sand used in this study was the product of several cycles of mechanical reclamation of the moulding material described above. It contained silica sand with a fair amount of (thermally) spent epoxy resin on the surface of the grains. Nevertheless, certain sections of a used mould/core positioned far from the liquid metal remain “thermally untouched” by hardly reaching even 100 °C. However, these parts likewise enter the reclamation process. Therefore, the reclaimed sand contained also fresh resin in addition to spent resin. As of appearance, reclaimed sand was also sub-rounded but relatively darker in colour compared to pure silica sand, due to the thermal effects after several casting cycles and to the presence of multiple spent resin layers. The sand grains tend to agglomerate, increasing the amount of coarser fractions and medium grain size to 0.28 mm, without the formation of fine particles as the dust debris is removed during the reclamation process (Fig. 3).
The most important parameter of the reclaimed sand besides adequate grain size distribution is LOI, which represents the amount of fresh and spent resin on the surfaces. The spent resin is from various stages of thermal decomposition and can significantly affect the thermal properties and the heat absorption behaviour of moulds and cores in case of their addition to the fresh moulding material. Typical LOI value of the reclaimed sand used in this work was 2.5 ± 0.1 mass%. Free moisture content was 0.02 mass%, much lower compared to the “base” mixture because the material was heated up several times to at least above room temperature. The reclaimed sand was also studied by TG–DTA in order to draw differences between the thermal behaviour of fresh and spent resin in the system.
DTA curve in Fig. 4 shows the diverse degradation mechanism of the reclaimed sand. The fresh resin started to burn out first, and endotherm peaks A and B representing this process appeared again, similar to the ones in Fig. 2. Meanwhile, the secondary/continued degradation of the spent resin also started. The TG curve shows that the mass loss taking place between 250 and 600 °C is much higher, compared to the mass loss in the “base” mixture (Fig. 2) during the same temperature interval. This means that the major decomposition processes of the spent resin took place between 250 and 600 °C, which is expected to establish additional heat absorbing processes in the moulding material. However, the static air atmosphere allowed the combustion of the spent resin and the degradation products, contrary to real foundry conditions where pyrolysis is dominant. Therefore, a major exothermic peak (C) appeared on the DTA curve between 450 and 500 °C. Endotherm peak “D” indicating the allotropic transformation of silica sand was less apparent this time, because of the shadowing of the exothermic peak. The transition of the TG curve at approximately 600 °C showed that the combustion of chemicals is finished until this temperature. Total mass loss value of ~2.5% corresponds well to the LOI result of reclaimed sand.
TG–DTA showed valuable initial results; however, the mixtures were further studied by Fourier thermal analysis to obtain understandings modelling real foundry conditions. These conditions were provided by the application of actual core wall thicknesses and heating rates prevalent in foundry technology.
Experimental
During sample preparation, clean silica sand was first mixed with reclaimed sand in different ratios shown in Table 3. Five different sand mixtures containing clean and reclaimed sand were then bonded by 1 mass% fresh resin and cured by SO2 gas. Production properties are given in Table 1.
The preparation of spherical sand samples made by mixtures A–E with different diameters of 40, 50, and 60 mm was slightly modified in order to apply the Fourier thermal analysis method at high temperatures analogous to cast iron production. N-type mineral insulated thermocouples with stainless steel sheath were used for temperature measurements; one temperature measuring point was in the geometrical centre of the cores, and another lateral measuring point was near the sample wall (Fig. 5). Exact locations of temperature reading points concerning all three sample diameters were akin to the dimensions used in a previous work (Table 4) [8]. Neither preliminary drying nor coating of the spheres was applied.
Samples were immersed into liquid cast iron (1300 ± 10 °C) during the measurement. Quartz glass pipes with outer diameter of 5 mm and wall thickness of 1 mm were used in the initial tests to avoid the direct contact of thermocouples with the melt during the immersion of the specimens. However, the thermocouple readings were disturbed due to the significant gas pressure built up inside the cores, which was a result of the more intense heat shock in the cast iron melt compared to the immersion into liquid aluminium in the earlier work. Therefore, the outer diameters of the protective pipes (ø p1 and ø p2) with a wall thickness of 1 mm (t1 and t2) were reconsidered as shown Table 5, to obtain an adequate evacuation of the gases from the samples.
Results of temperature measurements
Figure 6 shows the temperature distribution versus time in the 50-mm-diameter samples in case of all five mixtures. There are significant differences between the heating characteristics recorded in the centre of a specimen and in the lateral measuring point. Central temperatures (Fig. 6a) increase much slower compared to the lateral positions (Fig. 6b). The difference (gradient) is also evident comparing 40- and 60-mm sample diameters (Figs. 9, 10 in Appendix 1).
Compared to the results from earlier tests [8], application of cast iron melt provided higher heating rates than using aluminium melt. For instance, reaching 500 °C in the centre of a 50-mm sample took approximately 140 s in cast iron melt, while it took 350–370 s in aluminium melt. However, only a lower heating rate in aluminium melt ensured the preliminary observation of several heat absorbing features on the primary heat distribution versus time curves. As shown in Fig. 6, neither heat absorbing processes, nor differences between various mixture systems could be marked squarely on the results of temperature measurements due to the higher heating rates obtained by using cast iron melt. This phenomenon enhances the role of the thermal analysis in the exploration of heat absorbing processes taking place in the epoxy-SO2 moulding mixtures with various reclaimed sand additions.
Results of Fourier thermal analysis
The Fourier thermal analysis (FTA) is mainly used in non-ferrous and ferrous foundries for process monitoring. Originally, the method is applied to determine the release of latent heat during solidification in metallic alloys. Its fundamentals are based on using at least two measuring points in a 1-dimensional thermal field and the tabulation of the volumetric heat capacity of the phases taking part in the solidification [8].
In this paper, the Fourier thermal analysis used in an inverse way to study heating curves recorded in core samples instead of the traditional way used for cooling curves was developed during earlier works and has been presented in recent papers [9,10,11]. The calculation of total absorbed heat, fraction of absorbed heat, and rate of heat absorption by the degradation of the moulding material gave valuable information about the cooling capacity of the epoxy-SO2 cores together with the effect of various levels of additional reclaimed sand.
The most important governing condition for the transformation from liquid to solid is the temperature gradient, which depends mainly on the heat transfer between the melt and the moulding material and takes place at the liquid metal–mould interface. The heat transfer is strongly affected by the cooling capacity of moulding materials. Mixtures with high heat absorption capacity can increase cooling rates, and mixtures with low heat absorption capacity will eventuate in low cooling rates. Application of moulding materials with various cooling capacity can change the formation of the initial casting skin, which is a key moment in the relevant stages of the solidification phenomenon and in the formation of penetration, blow hole, or even shrinkage-related casting defects.
The calculated total absorbed heat values of all five mixtures are given in Table 6. Nearly equal result regardless of the sample diameter is an evidence of the good reproducibility of the method. Total absorbed heat means the heat necessary for the overlapping decomposition processes and phase transitions to take place, which were studied through the TG–DTA (Figs. 2, 4). These are the vaporization of free moisture content in the “base” mixture and in the reclaimed sand at 100 °C, the degradation of the fresh resin between 150 and 550 °C, the secondary or additional decomposition of the spent resin up until 600 °C, and the transformation of silica sand from α-quartz to β-quartz at 573 °C.
Results showed that 25 mass% of additional reclaimed sand content (e.g. the surplus of both fresh resin and the still combustible spent resin in the system) increased the total absorbed heat by approximately 10–12%. This means that the reclaimed sand eventually increased the cooling capacity of the cores, which is expected to shorten the total solidification time in the casting, significantly affecting its final microstructural morphology and may result in a large variations in mechanical properties.
Earlier authors also investigated the effect of various mould materials on the cooling rate of cast iron castings [12]. They only distinguish metallic, sand, ceramic, and insulated moulds. According to their findings, the application of these materials may eventuate in more than 60 °C difference in the temperatures at the end of solidification. At the same time, other works primarily focused on different chemically bonded sand moulds and concluded the significance of the type of sand on the final microstructure and mechanical properties of aluminium alloys [13]. Other works dealing with the thermophysical properties of green sand concluded the importance of different ingredients on the thermal conductivity of moulds [14,15,16].
Thus, the method suitable to study the effect of a specific mixture parameter (such as reclaimed sand content) on the heat absorption behaviour of a particular mixture system (epoxy-SO2) is of high importance from a thermal science point of view. By the right of the inverse thermal analysis, the effect of spent resin level on the cooling capacity can be further evaluated versus the temperature in the specimens. For this purpose, fraction of total absorbed heat and rate of heat absorption were also calculated.
Figure 7 shows the fraction of total absorbed heat versus the temperature recorded in the centres of the 50-mm-diameter cores. The effects of additional reclaimed sand appeared clearly, as the fractions of the total absorbed heat in the mixtures with different additional reclaimed sand can be assigned to a certain temperature in the centre of the sample. For instance, approximately 70% of the total absorbed heat was consumed by mixture A (without reclaimed sand) at 200 °C, which corresponded to ~100 kJ. The same amount of heat was absorbed by mixture E (100% reclaimed sand) up to 200 °C, but in this case, it corresponded to a much smaller part of the total heat absorbed, around 45%. This shows that the additional reclaimed sand level in the mixture increased the heat absorption starting from temperatures even lower than 200 °C, as the surplus of fresh resin in the reclaimed sand has already started to decompose by then. As the temperature increased, the effect of the secondary/continued degradation of spent resin became more and more dominant. As shown in Figs. 7 and 11 in Appendix 2, decomposition processes were completed until 550–600 °C regardless of sample diameter, confirming the results of TG–DTA.
Figure 8 shows the rate of heat absorption versus the temperature recorded in the centres of the 50-mm-diameter cores. These curves represent the degradation characteristics of each mixture variables. Rate of heat absorption reached a maximum shortly after 100 °C in all five mixtures. The amount of additional reclaimed sand did not affect this maximum peak of free moisture vaporization, because reclaimed sand did not add significant surplus of free moisture to the mixture. On the other hand, maximum rate of heat absorption was strongly affected by the heating rate, e.g. the sample diameter. This dependence is presented in Table 7, according to the curves in Figs. 8 and 12 in Appendix 2.
Thus, the level of additional reclaimed sand did not have a significant impact on the initial degradation processes of the epoxy-SO2 mixtures and therefore will not influence the very early stages of casting solidification. However, the extra amount of still degradable fresh and spent resin in the system played a significant role at higher core temperatures. Figure 8 also shows that mixtures B–E containing reclaimed sand had several secondary maximum peaks in the core temperature interval of 250–600 °C due to the secondary/continued degradation of spent resin. Moreover, curves of mixtures B–E generally had higher heat absorption rates, compared to mixture A with no additional reclaimed sand. This confirms the dominance of spent resin degradation at higher core temperatures, which is expected to prolong the cooling capacity of the moulds or the cores. This phenomenon will affect the solidification and the microstructure formation of the castings, as the moulds and cores containing reclaimed sand will still have a strong cooling capacity much longer after the pouring.
Conclusions
In this work, the effect of reclaimed sand addition on the cooling capacity of epoxy-SO2 mixtures was studied. TG–DTA was carried out to gain basic information about the thermal decomposition of the epoxy-SO2 system and the reclaimed foundry sand, respectively. TG–DTA presented valuable initial results; however, the materials were further examined in real foundry conditions by the novel application of Fourier thermal analysis described in a previous paper. The method of sample preparation was modified to run temperature measurements and Fourier thermal analysis in spherical epoxy-SO2 sand specimens at temperatures according to their every day application in cast iron production (1300 ± 10 °C).
The results of primary measurements enhanced the role of thermal analysis at temperatures of cast iron production, because no clear conclusions about the heat absorbing processes could be made based only on the heating curves because of higher heating rates.
The calculated total absorbed heat values showed that 25 mass% of additional reclaimed sand content increased the total absorbed heat by mixture decomposition by approximately 10–12%. This means that the combustible materials on the surface of the reclaimed sand will improve the cooling capacity of the cores. This phenomenon reflects on the possibility of controlling the solidification time in a casting simply by reusing mechanically reclaimed epoxy-SO2 mixture.
By the right of calculation of the fraction of absorbed heat and rate of heat absorption, the effect of reclaimed sand level on the cooling capacity can also be evaluated versus the temperature in the core specimens.
The fraction of absorbed heat curves indicated that the additional fresh and spent resin in the system improved the cooling capacity of the moulding material at a wide temperature range (150–600 °C). This will affect the solidification and the microstructure formation of the castings, as the moulds and cores containing reclaimed sand will have improved cooling capacity much longer after the pouring.
The rate of heat absorption results showed that additional reclaimed sand did not influence the heat absorption by the vaporization of moisture content and the early stages of mixture degradation. On the other hand, this was strongly affected by the heating rate, e.g. the sample diameter or the temperature of the melt. The curves underlined that the presence of spent resin in the mixture will predominantly improve the cooling capacity of the moulds or the cores at higher temperatures.
The outcome of the paper reflects on the future possibility of controlled solidification achieved by return sand addition to the epoxy-SO2 mixture and contributes to the topics of thermal sciences, simulation of casting processes, and also foundry technology in general.
References
Kroker J, Shiver R, Wang X. Customized SO2 curable cold box binders. Trans Am Foundry Soc. 2004;112:623–8.
Liu Q, Bao X, Deng S, Cai X. The investigation of methyl phenyl silicone resin/epoxy resin using epoxy-polysiloxane as compatibilizer. J Therm Anal Calorim. 2014;118:247–54.
ASM metals handbook volume 15: castings. 4th edition. ASM International, Metals Park, OH; 1998. p. 212–21.
Chemically bonded cores and molds. American Foundry Society, Schaumburg, IL; 1987. p. 113–8.
Zhang H, Zhao H, Zheng K, Li X, Liu G, Wang Y. Diminishing hazardous air pollutant emissions from pyrolysis of furan no-bake binders using methanesulfonic acid as the binder catalyst. J Therm Anal Calorim. 2014;116:373–81.
Principles of sand control. Section IX - Sand reclamation, American Foundry Society, Green Sand Committee 4-M, Schaumburg, IL; 2004.
Danko R, Holtzer M, Danko J. Investigations of physicochemical and thermal utilisation of dusts generated in the mechanical reclamation process of spent moulding sands. Arch Metall Mater. 2015;60(1):313–8.
Svidró JT, Diószegi A, Tóth J. The novel application of Fourier thermal analysis in foundry technologies. J Therm Anal Calorim. 2014;115:331–8.
Diószegi A, Svensson IL. Interpretation of solidification by thermal analysis of cooling rate. Trans Indian Inst Met. 2005;4(8):611–6.
Diószegi A, Hattel J. Inverse thermal analysis method to study solidification in cast iron. Int J Cast Met Res. 2004;17(5):311–8.
Diószegi A, Diaconu VL, Fourlakidis V. Prediction of volume fraction of primary austenite at solidification of lamellar graphite cast iron using thermal analyses. J Therm Anal Calorim. 2016;124:215–25.
Stan S, Chisamera M, Riposan I, Barstow M. Application of thermal analysis to monitor the quality of hypoeutectic cast irons during solidification in sand and metal moulds. J Therm Anal Calorim. 2012;110:1185–92.
Guanglei L, Naichao S, Shaochun S, Qinfang W. Effects of different casting mould cooling rates on microstructure and properties of sand-cast Al–7.5Si–4Cu alloy. China Foundry. 2013;10(6):396–400.
Krajewski P K, Piwowarski G. Range of thermal conductivity changes of wet green foundry sand during casting solidification. Arch Metall Mater. 2015;60(3):2391–5.
Krajewski P K, Piwowarski G, Zak P L, Krajewski W K. Experiment and numerical modelling the time of plate-shape casting solidification vs. thermal conductivity of mould material. Arch Metall Mater. 2014;59(4):1405–8.
Krajewski P K, Zovko-Brodarac Z, Krajewski W K. Heat exchange in the system mould-riser-ambient. Pt. 1, Heat exchange coefficient from mould external surface. Arch Metall Mater. 2013;58(3):833–5.
Acknowledgements
The present work was financed by the Swedish Knowledge Foundation. Cooperating parties in the project were Jönköping University, Scania CV AB and Volvo Powertrain Production Gjuteriet AB. External contribution was provided by the University of Miskolc. Participating persons from these institutions/companies are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Appendix 2
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Svidró, J.T., Diószegi, A., Svidró, J. et al. Thermophysical aspects of reclaimed moulding sand addition to the epoxy-SO2 coremaking system studied by Fourier thermal analysis. J Therm Anal Calorim 130, 1779–1789 (2017). https://doi.org/10.1007/s10973-017-6612-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6612-x