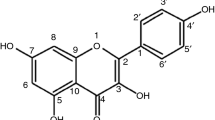
Abstract
Application of thermogravimetry (TG) alone to study compatibility/incompatibility of active pharmaceutical ingredients (APIs) with excipients yields to misleading results due to overlapping of the thermal stages in the course of decomposition of both ingredients and their pharmaceutical mixtures. Hence, the purpose of this study was to assess the usefulness of multivariate statistical analysis as a supporting tool for interpretation of the TG traces during assessing compatibility of hydrocortisone as an API with selected excipients (mannitol, starch, lactose, methylcellulose, β-cyclodextrin, meglumine, chitosan, magnesium stearate and polyvinylpyrrolidone). The results show that two multivariate techniques, principal component analysis (PCA) and cluster analysis (CA), can be successfully used for interpretation of TG traces, while the TG is used alone as a screening technique to assess compatibility. The results obtained by using TG analysis, supported by PCA and CA, were approved by those of differential scanning calorimetry, infrared spectroscopy and X-ray powder diffraction. Incompatibilities were only found in mixtures of hydrocortisone with magnesium stearate and β-cyclodextrin.
Similar content being viewed by others
Introduction
Preformulation stage of solid dosage formulations includes detection of possible interactions of an active pharmaceutical ingredient (API) with excipients [1–4]. Chemical interactions result in reduction of API quantity in the formulation that can lower its absorption and therapeutic effect. Physical interactions can alter physicochemical properties of the active ingredient, such as solubility, dissolution rate and finally bioavailability [5, 6]. There is no universally accepted protocol in the literature or issued by regulatory agencies for evaluating API–excipient compatibility or interactions [7, 8]. Methods for robust characterisation of API–excipient interactions are limited, time-consuming and labour intensive due to the number of variables that will need to be incorporated into the study, e.g. isothermal stress testing (IST) or HPLC. Hence, for compatibility tests the commonly recommended are techniques of thermal analysis.
Thermogravimetry (TG) is one of the thermal methods that has been used to detect compatibilities of API and excipients [9]. This technique is commonly applied to the study of thermal decomposition providing information on thermal stability of pharmaceutical substances. However, TG does not provide information on reactions that do not involve mass change, such as polymorphic transformations and double-decomposition reactions [10]. Moreover, it is also useless for identification of a substance or a mixture of substances unless temperature range of the reaction has already been established and there are no interfering reactions. In the case of identified substances, the TG by its nature is a quantitative technique and can frequently be used to quantify a substance in a mixture. Regardless of that, to gain a complementary information about thermal events, which have been associated with mass loss (e.g. degradation) and those which have not (e.g. melting or crystallisation), the TG traces are routinely registered simultaneously with differential thermal analysis (DTA) or differential scanning calorimetry (DSC) scans [11–13].
In the case of incompatibility study, TG allows a direct comparison of thermal profiles of API and excipients with those of their mixtures [9]. Unfortunately, these comparisons do not provide unequivocal data about compatibilities, because crucial information that can be extracted from the shape of TG traces is the change of mass (loss or gain) during thermal decomposition and the temperature range within which this process occurs. Hence, full information about the character of thermal processes cannot be derived from the TG traces and a plain detection of incompatibilities of API with excipients can be misleading. Therefore, whenever possible, other thermal techniques such as DSC or DTA and non-thermal ones such as infrared spectroscopy (IR) and X-ray powder diffraction (XRPD) should be used in conjunction with TG.
Taking all above into consideration, the purpose of this study was to verify the usefulness of advanced multivariate statistical analysis for interpretation of TG traces when the TG is used alone as a screening technique for assessing compatibility of hydrocortisone with some excipients. Preliminary studies revealed that application of TG traces alone to detect incompatibilities in the mixtures of hydrocortisone with excipients yields misleading results due to overlapping of the thermal stages in the course of decomposition of both the ingredients and their mixtures. Hence, it is difficult to observe changes in thermal profiles of binary mixtures as compared to those of particular components this precluding a straightforward identification of compatibility in the pharmaceutical mixtures. Therefore, the techniques of unsupervised multivariate statistical analysis such as principal component analysis (PCA) and cluster analysis (CA) were used as supporting tools for interpretation of the TG traces during assessing incompatibility of hydrocortisone as representative of APIs with excipients such as mannitol, starch, lactose, methylcellulose, β-cyclodextrin, meglumine, chitosan, magnesium stearate and polyvinylpyrrolidone (PVP-30). The basis for multivariate calculations were data sets including the temperatures of subsequent mass losses obtained from TG traces of API, excipients and their mixtures. These temperatures are called variables, and the samples that have similar thermal characteristics will be located near each other in multidimensional space forming consistent group. Hence, these multivariate techniques enable to understand the relationships between variables and make it possible to discriminate or classify each sample. This way, the compatibility/incompatibility between ingredients is detected. Furthermore, to confirm the results obtained by TG combined with PCA and CA, complementary techniques such as DSC, FTIR and XRPD were used.
It is worth mentioning that some attempts have recently been made to use advanced statistical analysis for the detection of pharmaceutical incompatibilities. For instance, Pearson’s correlation coefficients (r) between the theoretical and experimental API–excipient FTIR spectra were calculated [14–16]. The deviation from unity was interpreted as an indication of problems. To sum up, Pearson’s correlation provided a more precise mathematical analysis of the FTIR spectra of the samples analysed during the compatibility study and could be considered as an auxiliary tool for interpretation of chemical interactions. Moreover, to improve the interpretation of the results extracted from the FTIR spectra of samples before and after heating in a DSC apparatus, PCA was also used [17]. After constructing an optimal PCA model, the residues left by each sample were evaluated. Samples located above the threshold line show significant changes in their spectra after heating, indicating latent incompatibilities. Because this strategy is more reliable than a visual comparison, in this paper a direct interpretation of the PCA scores scatter plot is presented as a simple, reliable and rapid tool for compatibility detection based on the TG traces.
Experimental
Hydrocortisone and excipients
Hydrocortisone was supplied by Pharma Cosmetic (Krakow, Poland). Starch and mannitol were obtained from POCh (Gliwice, Poland). Methylcellulose was purchased from the Shin-Etsu Chemical Co. (Tokyo, Japan), while lactose was supplied by BUFA BV (Uitgeest, The Netherlands). Chitosan, meglumine, β-Cyclodextrin and polyvinylpyrrolidone (PVP-30) were provided by Fluka (Siegen, Germany), whereas magnesium stearate was purchased from Faci (Carasco Genoa, Italy). All materials used in this study were of pharmacopoeial purity and were used as obtained.
Thermal methods
TG and DTG traces of the API, excipients and their mixtures were recorded using of a model OD-103 derivatograph (MOM, Budapest, Hungary) over the range between 25 and 700 °C. 200-mg samples placed in four flat-bottomed platinum pans were heated in air at a rate of 5 °C min−1. Alumina was used as a reference material.
DSC scans were performed using a heat-flux Mettler Toledo instrument (Model DSC 822e, Schwerzenbach, Switzerland) with samples of approximately 5 mg weighed into flat-bottomed aluminium pans. Scans were obtained at a rate of 10 °C min−1 in the range from 20 to 350 °C, using nitrogen as a purging gas at a flow rate of 70 mL min−1. A STARe software was used for the DSC scans analysis.
Infrared spectroscopy
IR spectra were recorded using a Carl Zeiss Jena instrument (Specord, Model M-80, Jena, Germany) using potassium bromide pellets, at room temperature in the range of 4000–200 cm−1. The ambient atmosphere was a background. Spectra Manager software was used for interpretation of the IR spectra.
X-ray powder diffraction
XRPD patterns were obtained using a D2 Phaser equipment (Bruker, Karlsruhe, Germany). A CuK α tube (k = 0.154060 nm), current of 10 mA and voltage of 30 kV were used. The samples were analysed under an exposure time of 0.10 s using a step size of 0.02° over the diffraction angle range 7–55° (2θ). The diffraction patterns were plotted using Diffrac.suite software.
Multivariate analysis
Statistica 10 software (StatSoft Inc., Tulsa, OK, USA) was used for PCA and CA calculations. Matrix of the data with dimensions of 7 × 8, where 7 is the number of rows (objects) and 8 is the number of columns (variables), was the starting point for calculations. In the matrix, the thermal parameters obtained from the TG and DTG traces for the analysed samples were used as the columns. The rows were API and excipients alone and their physical mixtures.
Results and discussion
TG traces of hydrocortisone, selected excipients (chitosan and magnesium stearate) and binary physical mixtures of both ingredients at different molar or mass ratios are presented in Figs. 1 and 2. A detailed examination of the shapes of TG traces and temperature ranges over which the stages of thermal decomposition occurred showed that the TG technique did not provide unequivocal data in the case of compatibility study between hydrocortisone and selected excipients. These findings are consistent with those of Mendonça et al. [9], who studied compatibility/incompatibility in the mixtures of hydroquinone and retinoic acid with nine excipients. They confirmed that if no interactions occur, the TG trace of the mixture presents behaviour of both ingredients individually. On the other hand, different TG profiles of the mixture from those observed for individual ingredients arouse suspicion that this indicates interactions. To eliminate subjectivity in evaluation of acquired TG traces, two techniques of unsupervised multivariate statistical analysis (PCA and CA) were used to support interpretation of TG profiles.
PCA and CA as supporting techniques
The aim of PCA is data matrix reduction when some correlations between the variables exist. In this way, new variables called principal components (PCs) are created which are linear combinations of the original variables [18]. The principal components are orthogonal (uncorrelated, independent) to each other and describe the variation in data matrix, among which the first principal component (PC1) accounts for most of the variation in the data set, the second (PC2) accounts for the next largest variation and so on. The variation in the data for nine matrices constructed for API and excipients used in this study are compiled in Table 1. As it can be seen, the results of PCA calculations are best visualised in a two-dimensional scores scatter plot, PC1 and PC2, in which both ingredients and their mixtures create distinctly separated clusters. CA, another unsupervised technique of multivariate statistical analysis, was performed as a continuation of PCA to assess whether using different classification algorithm, a more sensitive classification of both ingredients and their mixtures can be expected. This technique evaluates the clustering tendency of samples through iterative process which associates the samples (agglomerative method) taking into account the Euclidean distance between pairs of samples and the Ward’s linkage criterion according to which samples or clusters are merged. The graphical visualisation of the samples’ classification is presented as a tree diagram.
Results of PCA and CA calculations are presented graphically in Figs. 3 and 4 and summarised in Table 2. To detect the compatibility of hydrocortisone with the excipients, a scattering of clusters in a plot of PC1 and PC2 was taken into consideration. Should not any interaction had occurred between the ingredients the PCA plot in Fig. 3a might have shown two distinctly separated clusters to be formed, the first located at the lowest values of PC1 including chitosan and a mixture with its high content, whereas the other located at the highest values of PC1 including hydrocortisone and the remaining mixtures, mostly with a high content of API. Distribution of all samples (API and the excipient alone and their mixtures) along the PC1 axis begins from the first ingredient alone found at the lowest value of PC1, through the mixtures with gradually diminishing quantities of the first ingredient and increasing content of the other, until the latter located close to the highest values of PC1 indicates that hydrocortisone and chitosan are compatible, i.e. between these ingredients no interaction occurs.
Similar scattering of the clusters was also observed in the case of mixtures of hydrocortisone with meglumine, PVP-30, lactose, starch, methylcellulose and mannitol. Three clusters were formed in each case. The first cluster is created by excipient alone (meglumine and PVP-30) or excipient and mixture(s) with the highest content of excipient (mixture in the 1:9 ratio or the 1:9 and 3:7 mixtures. Another cluster consisted mostly of mixtures of both ingredients in the ratios of 3:7, 1:1 and 7:3. The last cluster includes hydrocortisone alone or hydrocortisone and mixtures with the highest content of API in the 9:1 ratio. Such a distribution of the sample scattering in the PC1 and PC2 plots shows that the ingredients are compatible and can be blended for pharmaceutical applications.
PCA scores scatter plot differs from the above scheme in the case of incompatibilities between ingredients. The samples are not distributed along the PC1 axis according to their chemical composition, i.e. the content of the first ingredient decreases with increasing quantities of the other, but create separate clusters composed of mixtures with a quite different content of both ingredients. This is shown in Fig. 4a where hydrocortisone is incompatible with magnesium stearate. In this case, the first cluster includes magnesium stearate and its 1:9 mixture with hydrocortisone. The other cluster consists of the 1:1 mixture of both ingredients, while the third one is created by hydrocortisone and mixtures with the API/excipient ratios of 9:1, 7:3 and 3:7. Incompatibility of hydrocortisone with β-cyclodextrin was also detected with the aid of PCA. This enabled to create three separated clusters—one corresponding to the 9:1 of hydrocortisone and β-cyclodextrin mixture, the second one formed by β-cyclodextrin and its two 1:9 and 3:7 mixtures with hydrocortisone. The third cluster comprises two mixtures of both ingredients in the 7:3 and 1:1 ratios of API to excipient.
Results of calculations obtained by using of CA shows that if ingredients are compatible, two large clusters are created between 33 and 66 % of the maximum distance. This is reflected by the CA tree diagram in Fig. 3b. The first cluster groups chitosan and its mixture with a high chitosan content, while another cluster consists of hydrocortisone and mixtures with its high content, with the API-to-excipient ratios 9:1, 7:3, 1:1 and 3:7. This is compatible with the results of PCA (Fig. 3a). Similar organisation of the clusters is also observed in the CA tree diagrams of binary mixtures of hydrocortisone with mannitol and lactose; excipient and the 1:9 API/excipient mixture create the first cluster, while the remaining mixtures and hydrocortisone form the second cluster. CA calculations for mixtures of API with meglumine and PVP-30 show that first cluster contains additionally a hydrocortisone/excipient 3:7 mixture. In the case of mixtures of hydrocortisone with methylcellulose and starch, the first group consists of hydrocortisone and the API/excipient 9:1 mixture, the second group—excipient and the remaining mixtures. Such an organisation of clusters indicates a close similarity of the TG profiles of the mixtures to those of the main ingredient (API or excipient), thus excluding chemical or physical interactions.
A comparison of the CA tree diagrams of mixtures of compatible ingredients with those of hydrocortisone with magnesium stearate and β-cyclodextrin shows different organisation of the cluster. Similarly as in the PCA for mixtures of incompatible ingredients, the clusters do not consist of all samples with similar chemical composition, but those with quite different composition. As shown in Fig. 4b, between 33 and 66 % of the maximum distance there are three clusters of mixtures of hydrocortisone with magnesium stearate. A mixture with the 3:7 of API/excipient ratio was found in the second cluster even though its chemical composition is similar to that of the 1:9 mixture and magnesium stearate which create the first cluster. A similar grouping shows the CA tree diagram of mixtures of hydrocortisone with β-cyclodextrin. The first cluster includes β-cyclodextrin and three mixtures with quite different composition in the API/β-cyclodextrin ratios of 1:9, 3:7 and 9:1. The second cluster is formed by the remaining mixtures, while the third cluster consists of hydrocortisone alone. This shows that hydrocortisone is incompatible also with β-cyclodextrin.
Those results indicate that PCA and CA are helpful as tools for interpretation of the TG traces. A correlation between chemical composition of samples and their thermal profiles is highlighted by clustering, thus removing subjectivity of the observations. It can thus be concluded that β-cyclodextrin and magnesium stearate are incompatible with hydrocortisone. Hydrocortisone has been found to form an inclusion complex with β-cyclodextrin [19–22]. Incompatibility of hydrocortisone with magnesium stearate is probably due to interaction of the –OH group of hydrocortisone with the bridging carboxylic group of magnesium stearate via hydrogen bonding involving water, similarly as in the case of captopril and magnesium stearate. The literature data show that there are numerous reports on incompatibility of stearates with active pharmaceutical ingredients [6].
DSC, IR and XRDP for verification of results
To verify the results obtained using TG supported by multivariate techniques, DSC scans, IR spectra and XRPD diffraction patterns were recorded for API, excipients and all of the mixtures of both components. In contrast to TG traces, the DSC scans were only acquired in the range from 20 to 350 °C because in that range an appearance or disappearance of one or more peaks in DSC profile of drug mixture with excipient, and a broadening and a shift towards lower temperature of the endothermic DSC peak of API owing to the melting, can be considered as an interaction indication. DSC scans (Figs. 5a, 6a, 7a) show endothermic peaks due to the melting of hydrocortisone at about 226.0 °C (melting with decomposition). Chitosan (Fig. 5g) shows broader endothermic peaks at 100.6 °C (dehydration) and 275.9 °C (glass transition) followed by exothermic peak at 305.1 °C (decomposition). DSC scans of the mixtures of hydrocortisone with chitosan show a DSC peak of hydrocortisone slightly shifted to a lower temperature (about 222 °C). This shows that there are no alterations in the thermal profile of hydrocortisone. Magnesium stearate (Fig. 6g) shows an endothermic peak due to the melting at 102.99 °C. In the DSC scans of the mixtures of hydrocortisone with magnesium stearate, a characteristic peak of hydrocortisone (melting at 226.0 °C) is missing. This result suggests that hydrocortisone and magnesium stearate are incompatible.
Particular attention should be paid to the DSC scans of hydrocortisone mixtures with lactose (Fig. 7). Disappearance of the DSC peak (Fig. 7b) due to the melting of API suggests incompatibility of both ingredients. On the other hand, no variations confirming chemical interactions were found in the IR spectra and XRPD diffraction patterns of these mixtures. A detailed inspection of the thermal events due to the melting of hydrocortisone (216 °C [23], 214 °C [24] or 221.5 °C [25]) and of anhydrous α-lactose (222.8 °C [26], 223 °C [27–29]) suggests that both endothermic peaks are superimposed excluding incompatibility.
The IR spectra of hydrocortisone, excipients and their mixtures are shown in Figs. 8 and 9. Inspection of these spectra shows that characteristic bands of ingredients alone are consistent with those reported in the literature [30–34]. There are characteristic bands of hydrocortisone due to stretching vibrations of the O–H groups, C–H bonds, C=O groups and C–OH bonds over the ranges 3650–3100, 3100–2800, 1850–1540 and 1260–1000 cm−1, respectively. The spectrum of chitosan (Fig. 8g) displays bands for stretching vibrations of O–H and N–H, C–H and C–OH bonds over the ranges 3600–3000, 3000–2850 and 1260–1050 cm−1, respectively. The bending vibrations of N–H bonds of this excipient emerged over the range 1650–1580 cm−1, while deformation vibrations of C–OH and C–CH bonds were found over the range of 950–700 cm−1. All of these characteristic bands of API and the excipient were found in the IR spectra of binary mixtures of both ingredients with intensities proportional to the quantity of ingredients in the mixtures. This indicates the absence of any chemical interactions between hydrocortisone and chitosan.
The IR spectrum of magnesium stearate (Fig. 9g) shows stretching vibrations of O-Mg, C–H and C=O bonds over the ranges 3650–3100, 3000–2840 and 1650–1550 and 1400 cm−1, respectively as well as bending vibrations of C–H bonds at 1450–1375 cm−1. The IR spectra of hydrocortisone mixtures with magnesium stearate display variations in the intensity of some characteristic bands of API over the range 1850–1700 cm−1 (stretching bands of C=O) and 1300–1000 cm−1 (stretching bands of C–OH), which are non-related to the API quantity in the mixtures. These changes imply that both ingredients are incompatible.
The results obtained using TG, supported by multivariate statistical analysis, were subsequently approved by XRPD. The diffraction patterns of hydrocortisone mixtures with chitosan and magnesium stearate (Figs. 10, 11) show all characteristic peaks similar to those of particular ingredients.
Conclusions
The results of this study show that the techniques of advanced multivariate statistical analysis, such as PCA and CA, can successfully be used for interpretation of TG traces, while the TG can be used alone as a screening technique to assess compatibility/incompatibility of the ingredients of pharmaceutical mixtures consisting of selected excipients, such as mannitol, starch, lactose, methylcellulose, β-cyclodextrin, meglumine, chitosan, magnesium stearate and PVP-30. A correlation between chemical composition of binary mixtures and their TG profiles is highlighted by clustering, thus removing subjectivity of the observations. Graphical interpretation of the PCA calculations shows that scattering of analysed samples in two-dimensional plane defined by PC1 and PC2 displays if there is compatibility/incompatibility between drug and excipient. The creation of two separate areas where one groups API and its mixtures with high API’s content, and the other, excipient and its mixtures with high excipient’s content, indicates compatibility between ingredients. Otherwise, incompatibility can be expected. A comparison of the CA tree diagrams with those of the PCA score biplots shows that the results obtained using both multivariate approaches are consistent. In conclusion, both PCA and CA make much of contribution to interpretation of the TG profiles of hydrocortisone binary mixtures with selected excipients. These findings can be very useful for preformulation study to develop the stable, effective and safe solid dosage formulations by establishing API compatibility with the other ingredients. The results obtained by using TG, supported by PCA and CA, were also approved by the DSC, IR and XRPD analyses of the same hydrocortisone binary physical mixtures with those excipients. Incompatibilities were only found in the case of API mixtures with magnesium stearate and β-cyclodextrin.
References
Fuliaş A, Ledeţi I, Vlase G, Popoiu C, Hegheş A, Bilanin M, Vlase T, Gheorgheosu D, Craina M, Ardelean S, Ferechide D, Mărginean O, Moş L. Thermal behaviour of procaine and benzocaine. Part II: compatibility study with some pharmaceutical excipients used in solid dosage forms. Chem Central. 2013;7:1–10.
Stulzer HK, Rodrigues PO, Cardoso TM, Matos JSR, Silva MAS. Compatibility studies between captopril and pharmaceutical excipients used in tablets formulations. J Therm Anal Calorim. 2008;91:323–8.
Sousa e Silva JP, Sousa Lobo JM. Compatibility studies between nebicapone, a novel COMT inhibitor, and excipients using stepwise isothermal high sensitivity DSC method. J Therm Anal Calorim. 2010;102:317–21.
Nunes RS, Semaan FS, Riga AT, Cavalheiro ETG. Thermal behavior of verapamil hydrochloride and its association with excipients. J Therm Anal Calorim. 2009;97:349–53.
Aigner Z, Heinrich R, Sipos E, Farkas G, Ciurba A, Berkesi O, Szabó-Révész P. Compatibility studies of aceclofenac with retard tablet excipients by means of thermal and FT-IR spectroscopic methods. J Therm Anal Calorim. 2011;104:265–71.
Bharate SS, Bharate SB, Bajaj AN. Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: a comprehensive review. J Excip Food Chem. 2010;1:3–26.
Adeyeye MCh, Brittain HG, editors. Preformulation in solid dosage form development. New York: Informa, Healthcare; 2008.
Verma RK, Garg S. Selection of excipients for extended release formulations of glipizide through drug-excipient compatibility testing. J Pharm Biomed Anal. 2005;38:633–44.
Mendonça CMS, de Barros Lima IP, Aragão CFS, Gomes APB. Thermal compatibility between hydroquinone and retinoic acid in pharmaceutical formulations. J Therm Anal Calorim. 2014;115:2277–85.
Kaufmann EN. Characterization of materials. Hoboken: Wiley; 2003.
Haines PJ. Thermal methods of analysis. Principles, applications and problems. Dordrecht: Springer; 1995.
Haines PJ. Principles of thermal analysis and calorimetry. Cambridge: The Royal Society of Chemistry; 2002.
Craig DQM, Reading M. Thermal analysis of pharmaceuticals. Boca Raton: Taylor & Francis Group; 2007.
de BarrosLima ÍP, Lima NGPB, Barros DMC, Oliveira TS, Mendonça CMS, Barbosa EG, Raffin FN, de Lima e Moura TFA, Gomes APB, Ferrari M, Aragão CFS. Compatibility study between hydroquinone and the excipients used in semi-solid pharmaceutical forms by thermal and non-thermal techniques. J Therm Anal Calorim. 2015;120:719–32.
de Barros Lima ÍP, Lima NGPB, Barros DMC, Oliveira TS, Barbosa EG, Gomes APB, Ferrari M, do Nascimento TG, Aragão CFS. Compatibility study of tretinoin with several pharmaceutical excipients by thermal and non-thermal techniques. J Therm Anal Calorim. 2015;120:733–47.
Lavor EP, Navarro MVM, Freire FD, Aragão CFS, Raffin FN, Barbosa EG, Moura TFAL. Application of thermal analysis to the study of antituberculosis drugs-excipient compatibility. J Therm Anal Calorim. 2014;115:2303–9.
Daniel JSP, Veronez IP, Rodrigues LL, Trevisan MG, Garcia JS. Risperidone-solid-state characterization and pharmaceutical compatibility using thermal and non-thermal techniques. Thermochim Acta. 2013;568:148–55.
Otto M. Chemometrics: statistics and computer application in analytical chemistry. 2nd ed. Weinheim: Wiley-VCH; 2007.
Swarbrick J. Encyclopedia of pharmaceutical technology. 3rd ed. New York: Informa Healthcare; 2007. p. 671–96.
Al-Sou’od KA. Investigation of the hydrocortisone-β-cyclodextrin complex by phase solubility method: some theoretical and practical considerations. J Sol Chem. 2008;37:119–33.
Forgo P, Göndös G. A study of β-cyclodextrin inclusion complex with progesterone and hydrocortisone using rotating frame overhauser spectroscopy. Monat Chem. 2002;133:101–6.
Miranda JC, Martins TEA, Veiga F, Ferraz HG. Cyclodextrins and ternary complexes: technology to improve solubility of poorly soluble drugs. Braz J Pharm Sci. 2011;47:665–81.
Polish Pharmacopoeia VI. Warszawa: Polskie Towarzystwo Farmaceutyczne; 2002.
Lund W. The pharmaceutical codex. 12th ed. London: The pharmaceutical Press; 2004.
Brittain H. Profiles of drug substances, excipients and related methodology. Vol. 12. Analytical profiles of drug substances, vol. 12. New York: The American Pharmaceutical Association, Academic Press; 1983.
Kibbe H. Handbook of pharmaceutical excipients. 3rd ed. London: American Pharmaceutical Association, Pharmaceutical Press; 2000.
Rowe RC, Sheskey PJ, Quinn ME. Handbook of pharmaceutical excipients. 6th ed. London: American Pharmaceutical Association, Pharmaceutical Press; 2009.
Holsinger VH. Lactose. In: Wong NP, Jenness R, Keeney M, Marth EH, editors. Fundamentals of dairy chemistry. New York: Van Nostrard Reinhold; 1988. p. 279–342.
Figura LO, Epple M. Anhydrous α-lactose: a study with DSC and TXRD. J Therm Anal. 1995;44:45–53.
Yazdi MT, Arabi H, Faramarzi MA, Ghasemi Y, Amini M, Shokravi S, Mohseni FA. Biotransformation of hydrocortisone by a natural isolate of Nostoc muscorum. Phytochemistry. 2004;65:2205–9.
Jia Z, Shen D, Xu W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr Res. 2001;333:1–6.
Ko YG, Sung BH, Choi US. Electrorheological properties of aminated chitosans. Colloids Surf A. 2007;305:120–5.
Stulzer HK, Tagliari MP, Cruz AP, Silva MAS, Laranjeira MCM. Compatibility studies between piroxicam and pharmaceutical excipients used in solid dosage forms. Pharm Chem J. 2008;42:215–9.
Silverstein RM, Webster FX, Kiemle DJ. Spectrometric identification of organic compounds. 7th ed. Hoboken: Wiley; 2005.
Acknowledgements
The investigations were financially supported by a statutory research, Grant No. ST-15, from the Ministry of Science and Higher Education, Poland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rojek, B., Wesolowski, M. Compatibility studies of hydrocortisone with excipients using thermogravimetric analysis supported by multivariate statistical analysis. J Therm Anal Calorim 127, 543–553 (2017). https://doi.org/10.1007/s10973-016-5441-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5441-7