Abstract
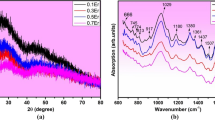
In the present report, physical and thermal characterizations of magnesium oxyhalide bismuth borate glasses in the composition range of \( x{\text{Mg}}X_{2} \left( {X = {\text{F}},\,{\text{Br}}} \right) \, \cdot \, \left( {30 - x} \right){\text{MgO }}\cdot \, 50{\text{B}}_{2} {\text{O}}_{3} \cdot \, 20{\text{Bi}}_{2} {\text{O}}_{3} \) (x = 0, 2, 5, 7 and 10) containing 2 mol% of V2O5 prepared by standard melt-quench technique have been discussed. The non-crystalline nature of prepared glasses has been identified through peak free X-ray diffraction profiles. Density (D), molar volume (V m) and oxygen packing density have been measured/calculated, and varying trends are discussed by relating with physical changes governed in prepared glass structures on increasing/changing the halogen (fluoride/bromide) ions. The differential thermal analysis (DTA) and thermogravimetry have been carried out in the temperature range of 200–900 °C. Glass transition temperature (T g) and melting temperature (T m) are determined from the DTA thermographs. The glass stability parameter, T g/T m is calculated from the obtained values of characteristic temperatures and a sudden decrease in stability at x = 5.0 is observed in both series which reflects that halide ions (F−/Br−) modify the glass structure at low concentrations (up to 5.0 mol%) and contribute to glass network formation at high concentrations (>5.0 mol%). Infrared absorption spectra have been recorded in the mid-IR range and evaluated to report the possible cause of band vibrations present in the spectra.







Similar content being viewed by others
References
Kashif I, El-Maboud AA, Ratep A. Effect of Nd2O3 on structure and characterization of lead bismuth-borate glasses. Results Phys. 2014;4:1–5.
Karthikeyan B, Mohan S. Structural, optical and glass transition studies on Nd3+-doped lead bismuth borate glasses. Phys B. 2003;334:298–302.
Ahlawat NN, Agamkar P, Ahlawat N, Agarwal A, Monica R. Structural study of TM doped alkali bismuth borate glasses. Adv Mater Lett. 2013;4:71–3.
Khasa S, Dahiya MS, Agarwal A. Effect of alkali addition on DC conductivity and thermal properties of vanadium-bismo-borate glasses. AIP Conf Proc. 2014;1591:796–8.
Ardelean I, Cora S, Rusu D. EPR and FT-IR spectroscopic studies of Bi2O3–B2O3–CuO glasses. Phys B. 2008;403:3682–5.
Khasa S, Dahiya MS, Ashima S, Agarwal A. Structural investigations of lithium vanadoxide bismo-borate glasses. J Integr Sci Technol. 2013;1:44–7.
Farouk H, Soliman AA, Farhan H, Kashif I, Sanad AM. Mössbauer, electrical, and magnetic studies of glass of the CaO–B2O3–Fe2O3–CaCl2 system. Can J Appl Spectrosc. 1996;41:135–8.
Doweidar H, El-Damrawi G, Abdelghani M. Structure and properties of CaF2–B2O3 glasses. J Mater Sci. 2012;47:4028–35.
Khasa S, Dahiya MS, Agarwal A. Synthesis and characterization of vanadyl ion doped alkaline halide borate glasses. Int J Phys Math Sci. 2012;2(S1):104–8.
Khasa S, Seth VP, Gahlot PS, Agarwal A, Krishna RM, Gupta SK. Electron paramagnetic resonance, optical transmission spectra and DC conductivity studies of vanadyl-doped alkali halide borate glasses. Phys B. 2003;334(3):347–58.
Khasa S, Dahiya MS, Agarwal A. FTIR studies of some vanadyl ion doped calcium oxychloride borate glasses. AIP Conf Proc. 2012;1536:671–2.
Khasa S, Dahiya MS, Agarwal A, Chand P. EPR, FTIR, thermal and electrical properties of VO2+ doped BaO·BaCl2·B2O3 glasses. J Mol Struct. 2015;1079:15–20.
Dahiya MS, Khasa S, Agarwal A. Optical absorption and heating rate dependent glass transition in vanadyl doped calcium oxy-chloride borate glasses. J Mol Struct. 2015;1086:172–8.
Dahiya MS, Khasa S, Agarwal A. Physical, thermal, structural and optical absorption of vanadyl doped magnesium oxy-chloride bismo-borate glasses. J Asian Ceram Soc. 2015;3:206–11.
Button DP, Tandon R, King C, Veléz MH, Tuller HL, Uhlmann DR. Insights into the structure of alkali borate glasses. J Non-Cryst Solids. 1982;49:129–42.
Sulowska J, Waclawska I, Olejniczak Z. Effect of glass composition on the interactions between structural elements in Cu-containing silicate-phosphate glasses. J Therm Anal Calorim. 2014;116:51–9.
Wers E, Oudadesse H. Thermal behavior and excess entropy of bioactive glasses and Zn-doped glasses. J Therm Anal Calorim. 2014;115:2137–44.
Sulowska J, Waclawska I, Szumera M. Effect of copper addition on glass transition of silicate–phosphate glasses. J Therm Anal Calorim. 2012;109:705–10.
Mošner P, Vosejpková K, Koudelka L, Beneš L. Thermal studies of ZnO–B2O3–P2O5–TeO2 glasses. J Therm Anal Calorim. 2012;107:1129–35.
Dalal S, Khasa S, Dahiya MS, Yadav A, Agarwal A, Dahiya S. Optical and thermal investigations on vanadyl doped zinc lithium borate glasses. 2000. doi:10.1016/j.jascer/2015.03.004.
Delben JRJ, Delben AAST, Miazato K, Oliveira SL, Messaddeq Y. Thermal stability of fluorochloroindate glasses. J Therm Anal Calorim. 2004;75:637–42.
Silva DV, Ribeiro CA, Crespi MS. Crystallization kinetic of fluoro-zirconate glasses by non-isothermal analysis. J Therm Anal Calorim. 2003;72:151–7.
Zhu J, Jin H, Dong D, Qiang D, Ma F. Study on the stability of GaF3-based glasses by differential scanning calorimetry. J Therm Anal Calorim. 2001;66:479–86.
Maraquesi AR, Delben JRJ, Delben AAST. Glass forming ability and thermal stability of oxyfluoride glasses. J Therm Anal Calorim. 2009;96:403–6.
Das SS, Singh P. DSC studies on sodium phosphate glasses doped with chlorides of Cd, Co and Ag. J Therm Anal Calorim. 2004;78:731–8.
Kiczenski TJ, Ma C, Hammarsten E, Wilkerson D, Affatigato M, Feller SA. J Non-Cryst Solids. 2000;272:57.
Feller SA, Lower N, Affatigato M. Density as a probe of oxide glass structure. Phys Chem Glasses. 2001;42:240–6.
Samee MA, Awasthi AM, Shripathi T, Bale S, Srinivasu Ch, Rahman S. Physical and optical studies in mixed alkali borate glasses with three types of alkali ions. J Alloys Compd. 2011;509:3183–9.
Dietzel A. Glass structure and glass properties. Glasstech. 1968;22:41.
Araujo EB, Eiras JA, de Almeida EF, de Paiva JAC, Sombra ASB. Structure and nucleation mechanism of the iron lithium niobium phosphate glasses studied by infrared spectroscopy and DTA. Phys Chem Glasses. 1999;40:273.
Sakka S, Mackenzie JD. Relation between apparent glass transition temperature and liquids temperature for inorganic glasses. J Non-Cryst Solids. 1971;6:145–62.
Hruby A. Evaluation of glass-forming tendency by means of DTA. Czech J Phys B. 1972;22:1187–93.
Saad M, Poulain M. Glass forming ability criterion. Mater Sci Forum. 1987;19:11.
Subbalakshmi P, Veeraiah N. Study of CaO–WO3–P2O5 glass system by dielectric properties, IR spectra and differential thermal analysis. J Non-Cryst Solids. 2002;298:89–98.
Kissinger H. Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand. 1956;57:217–21.
Mitrovic N, Roth S, Eckert J. Kinetics of the glass transition and crystallization process of Fe72−x Nb x Al5Ga2P11C6B4 (x = 0, 2) metallic glasses. Appl Phys Lett. 2001;78:2145–7.
Souri D. Investigation of glass transition temperature in (60−x)V2O5–40TeO2–xNiO glasses at different heating rates. J Mater Sci. 2011;46(21):6998–7003.
ElBatal FH, Marzouk SY, Nada N, Desouky SM. Gamma-ray interaction with copper-doped bismuth-borate glasses. Phys B. 2007;391:88–97.
Giridhar A, Mahadevan S. The T g versus Z dependence of glasses of the Ge–In–Se system. J Non-Cryst Solids. 1992;151:245–52.
Rabinal MK, Sangunni KS, Gopal ESR. Chemical ordering in Ge20Se80−x In x glasses. J Non-Cryst Solids. 1995;188:98–106.
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976;A32:751–67.
Singh L, Thakur V, Punia R, Kundu RS, Singh A. Structural and optical properties of barium titanate modified bismuth borate glasses. Solid State Sci. 2014;37:64–71.
Baia L, Stefan R, Kiefer W, Popp J, Simon S. Structural investigations of copper doped B2O3–Bi2O3 glasses with high bismuth oxide content. J Non-Cryst Solids. 2002;303:379–86.
Pascuta P, Borodi G, Culea E. Structural investigations of bismuth borate glass ceramics containing gadolinium ions by X-ray diffraction and FTIR spectroscopy. J Mater Sci Mater Electron. 2009;20:360–5.
Kamitsos EI, Karakassides MA, Chryssikos GD. Vibrational spectra of magnesium–sodium–borate glasses. 2. Raman and mid-infrared investigation of the network structure. J Phys Chem. 1987;91:1073–9.
Mansour E. FTIR spectra of pseudo-binary sodium borate glasses containing TeO2. J Mol Struct. 2012;1014:1–6.
Shaaban ER, Shapaan M, Saddeek YB. Structural and thermal stability criteria of Bi2O3–B2O3 glasses. J Phys Condens Matter. 2008;20:155108.
Acknowledgements
This work was supported by University Grant Commissions, New Delhi, under Major Research Project (F. No. 40-461/2011(SR)) and Department of Science and Technology, New Delhi, under INSPIRE Fellowship No. IF130355. Thermal measurement facilities were provided by Central Instrumentation Laboratory at Deenbandhu Chhotu Ram University of Science and Technology, Murthal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dahiya, M.S., Khasa, S. & Agarwal, A. Thermal characterization of novel magnesium oxyhalide bismo-borate glass doped with VO2+ ions. J Therm Anal Calorim 123, 457–465 (2016). https://doi.org/10.1007/s10973-015-4913-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4913-5