Abstract
Glass transformation effect of mixed SiO2–P2O5–K2O–MgO–CaO–CuO glasses was studied by DSC, XRD, SEM, and Raman spectroscopy methods. The relationship between the parameters characterizing glass transformation effect and an amount of phosphorous and copper forming the glassy structure was discussed. It was shown that an increasing content of phosphorous increased solubility of copper in the structure of the studied glasses which was the result of P–O–Cu bonds formation. Degree of changes of T g, ∆c p, and time of relaxation values were higher in glasses with higher content of P2O5 and CuO. The observed relations were explained on the basis of the local atomic interactions in the structure of glass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vitreous substances during heating and cooling exhibit glass transformation effect which is induced by relaxation of strains being the consequence of disordered arrangement of atoms forming glass structure. The relaxation of strains in the glass structure taking place at transformation temperature (T g) is related to a change of such properties as heat capacity, linear and volume expansion coefficients, and viscosity. Parameters characterizing the glass transformation effect depend on the nature and the number of components forming the glassy structure.
Copper may exist in glass at different oxidation states namely in the form of Cu2+, Cu+, and Cu0. This element is one of the glass structure modifiers. However, the introduction of excessive amount of copper into glass structure or wrong proportions of copper in relation to other components may facilitate crystallization of formed melt.
According to the literature glasses from (50−x/2)Na2O–xCuO–(50−x/2)P2O5 system, where x is less than 34 mol% [1], as well as glasses from 50P2O5–20Na2O–30CuO system [2] were totally amorphous. For glasses from P2O5–Na2O–CuO system the glass formation region was determined as P2O5 ≥ 40, CuO ≤ 50, and Na2O ≤ 60 mol% [3]. Almost all glasses from xCu2O–yP2O5–zMoO3 system, where x, y, and z changed from 0 to 50 mol% were characterized by high glass forming ability with the exception of glasses with low P2O5 content where the ratio of Cu2O/MoO3 was equal to unity because during cooling of their melt crystallized molybdates such as Cu2MoO4 and Cu2Mo2O7 [4]. Glasses from (0.70−x)SiO2–0.30Na2O–xCuO system where x changed from 0 to 0.2 mol% were completely amorphous [5]. In the case of glasses from B2O3–SiO2–Al2O3–CaO–CuO system boron oxide increased solubility of CuO in their structure. 15 mol% of B2O3 and 15–25 mol% of CuO in their structure caused crystallization of fine-needle CuO crystals during their synthesis. However, if B2O3 content was greater than 20 mol% these glasses did not show any tendency to crystallize during the synthesis [6].
In the literature, we can find information on the effect of copper on the glass transition effect of phosphate and silicate glasses. It was found that addition of increasing amount of CuO at the cost of NaO and P2O5 in structure of glasses from (50−x/2)Na2O–xCuO–(50−x/2)P2O5 system, where x is less than 34 mol% [1], as well as glasses from P2O5–Na2O–CuO system [3] caused increase of the glass transition temperature T g due to depolimeryzation of the glass network on smaller units which in turn combined by P–O–Cu bonds and resulted in an increase of crosslinking density of the glasses. According to the literature the ratio of Cu2+ ions to total amount of copper ions in the structure of glasses from 50P2O5–20Na2O–30CuO [2] and xCuO–(1−x)P2O5 (0 ≤ x ≥ 0.50) [7] systems had impact on their T g. The higher the ratio, the higher value of T g of these glasses.
The introduction of increasing amount of Cu2O to the structure of glasses from xCu2O–yP2O5–zMoO3 system where x, y, and z were from 0 to 50 mol% range, resulted in a decrease of the glass transition temperature (T g) because of formation of weaker ionic bonds Cu+–O at the expense of strong covalent P–O and Mo–O bonds [4].
Increase of copper content in the structure of glasses from (0.70−x)SiO2–0.30Na2O–xCuO system where x was from 0 to 0.2 mol% resulted in decrease of their T g [5].
The method of synthesis of glasses from Cu2O–Na2O–Al2O3–SiO2 system also had impact on the value of the glass transition temperature (T g) [8, 9]. Synthesis of these glasses using Cu+/Na+ ion-exchange treatment enabled to regulate the valence of copper ions in the structure of such glasses. The more Cu+ ions were exchanged in the course of their synthesis by ion exchange, the lower was the value of T g of these glasses.
However, there was no information on the effect of copper on glass transition of mixed silicate–phosphate glasses.
The object of these studies was silicate–phosphate glasses from SiO2–P2O5–K2O–MgO–CaO–CuO system which can act as slow-dissolving fertilizers providing plant macroelements (P, K, Mg, and Ca) [10], as well as copper acting as a microelement. Copper plays an active role in many life processes of plants, such as photosynthesis, respiration, metabolism of nitrogen compounds, the formation of proteins, carbohydrate transport, and formation of RNA and DNA [11].
The purpose of this study was to investigate the influence of CuO content on the glass forming ability and glass transition behavior of glasses from SiO2–P2O5–K2O–MgO–CaO system.
Experimental
Two groups of silicate–phosphate glasses from SiO2–P2O5–K2O–MgO–CaO–CuO differing in content of P2O5 and CuO were prepared. In both groups constant quantities of K2O and SiO2 were kept, and the increasing amount of CuO was introduced at the cost of decreasing amount of MgO and CaO, with the constant MgO/CaO ratio.
The silicate–phosphate glasses were produced by melting raw materials mixture, i.e., SiO2, H3PO4, MgO, K2CO3, CaCO3, and CuO at 1450 °C. Then the batch-free glasses were fritted in water. The obtained glasses were grinded to the grain size of 0.1–0.3 mm.
Chemical composition of the synthesized glasses was determined by the X-ray fluorescence spectrometry (XRF) method, using ARL ADVANT’XP spectrometer and was presented in Table 1.
All glasses were subjected to differential scanning calorimetry (DSC) measurements conducted on Netzch STA 449 F1 Jupiter, operating in the heat flux DSC mode. Glass samples weighing 60 mg were heated in platinum crucibles at a rate of 10 °C min−1 in a dry nitrogen atmosphere up to 1,100 °C. Characteristic temperatures of the glass transformation effect such as the onset and the end of the glass transformation region, the glass transformation temperature T g, determined as the midpoint of the cp, changes in the glass transformation region and changes of specific heat (∆c p) at T g points were determined applying the Netzsch Proteus Thermal Analysis Program (version 5.0.0.). Approximated relaxation time for the glass transformation τ = (T end−T onset)β−1 where β is the heating rate [12] was evaluated.
The X-ray diffraction method (X’Pert PRO Diffractometer, Philips) was used to confirm the amorphous state of the samples.
The scanning electron microscopy (SEM) was used to find out the microstructure of the analyzed glasses.
The micro-Raman spectroscopy study of glasses was carried out on Raman Jobin–Yvon T-64000 spectrometer, using 514.5 nm line of Ar laser (30 mW) for excitant. The spectra were recorded in the 1,400–200 cm−1 range of Raman shifts.
Results and discussion
Glass forming ability
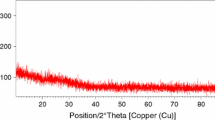
XRD results showed (Fig. 1a) that glasses with higher P2O5 content containing less than 18 mol% of CuO were completely amorphous. In the case of higher content of CuO in the structure of these glasses, XRD diffractograms revealed the presence of Cu2O in their structure.
Results of microstructure studies of the glasses with higher content of P2O5 with 6.5 and 18 mol% of CuO showed the presence of spherical inclusions in an amorphous matrix (Fig. 2a, b), which in the presence of higher content of CuO (35Cu6P) take the form of dendritic crystals of Cu2O (Fig. 2c).
On the other hand, in the case of glasses with a low P2O5 content and just 6 mol% of CuO a weak reflex on their diffraction pattern was observed which corresponded to the beginning of copper excess precipitation from the melt (Fig. 1b).
Thus, the higher content of P2O5 in the structure of the studied glasses, the higher solubility limit of CuO. This behavior suggested that copper in the silicate–phosphate glass structure formed Cu–O–P bonds.
Effect of copper as the glass modifier on structure of glasses with higher content of P2O5 was evidenced by Raman spectra (Fig. 3). They were characterized by bands at 960 and 1,070 cm−1 which could be assigned to Si–O− stretching vibrations in Q 2, Q 1 (at 960 cm−1), and Q 3 (at 1,070 cm−1) units in the silicate network. The effect of copper addition on this part of the spectrum was not very noticeable. On the other hand, the very weak band at 454 cm−1 for 0Cu6P glass sample could be attributed to symmetric stretching of O–P–O bending modes of the orthophosphate PO4 3− units (Q 0) which shifted towards lower wavenumber with the increasing content of CuO in the glass structure, indicating depolymerization of the phosphate network [13].
Sharper than the previous one band at 588 cm−1 for 0Cu6P glass sample could be attributed to the symmetric stretching of P–O− bonds of the orthophosphate units (Q 0). This band become more distinct with the increasing content of CuO in the structure of analyzed glasses.
The above observations confirmed that some changes around P–O–P bonds in the structure of the considered glasses took place. They might be related to formation of P–O–Cu bonds. Formation of such bonds can be explained based on the difference of the ionicity of bonds in the oxygen bridge (∆i G), which can be used to measure local chemical affinity of cations to the oxygen in oxide bridge. The higher is its value, the greater is the local chemical affinity of cations to the bridging oxygen [14].
Thus, the presence of copper in the structure of the studied glasses caused a break of P–O–P bonds rather than Si–O–Si bonds due to its greater affinity to phosphorus than to silicon (Cu2+–O–P ∆i G = 0.303 > Cu2+–O–Si ∆i G = 0.189, Cu+–O–P ∆i G = 0.449 > Cu+–O–Si ∆i G = 0.335).
Thermal studies
DSC curves of the studied glasses (Fig. 4a, b) showed transformation and crystallization process induced by the thermal treatment. Values of parameters characterizing the glass transformation were presented in Table 2.
It can be noticed that, the introduction of increasing amount of copper into the structure of completely amorphous network (2.5Cu6P, 5Cu6P, 1.5Cu2P, and 3Cu2P) caused broadening of temperature range of the transformation (i.e., increase of the relaxation time), decrease of the characteristic temperature T g and increase of ∆c p accompanying the glass transformation effect. Such behavior of glasses was observed in the case of silicate–phosphate glasses containing an increasing amount of Fe2O3 [15] and B2O3 [16], and it was the indication of an increasing degree of their structure relaxation.
On the DSC curve of glass containing 6.5 mol% of CuO (6.5Cu6P) the double endothermic glass transformation deflection (Fig. 4b) was visible. It was related to phase separation process (liquation) of amorphous matrix of the glass, which was observed in SEM investigations (Fig. 2a, b), and which proceeded the crystallization of Cu2O (Fig. 2c).
The structural relaxation of the partially recrystallized glasses was accompanied by changes of the specific heat ∆C p which values diminished (18Cu6P) and finally completely disappeared (35Cu6P).
The mentioned above changes of the parameters characterizing glass transformation were more visible in the glasses with higher content of P2O5.
Changes in the glass transformation temperature T g could be explained based on the nature of chemical bonds in the structure of glasses. The ionicity (i G) value of bonds of the component atoms with oxygen according to Görlich’s scale [17] were applied as a parameter characterizing the strength of bonds. The more covalent character of Cu+2–O bonds (i G = 0.617) replacing the more ionic bonds such as Ca–O bonds (i G = 0.707) and Mg–O bonds (i G = 0.670) caused the glass structure more rigid which consequence was increasing amount of stress (L Cu+2–O = 1.973) in the glass, which relaxation required less energy and hence lower values of T g but higher value of relaxation time.
Complex multistage crystallization processes induced by the thermal treatment in the multicomponent SiO2–P2O5–K2O–MgO–CaO–CuO glasses with variable copper content were presented in [18].
Conclusions
The appearance of glass transformation in mixed SiO2–P2O5–K2O–MgO–CaO–CuO glasses and magnitude of parameters characterizing this effect depended on phosphorous and copper content in the glassy structure. The increasing content of phosphorous caused increase of solubility of copper in the structure of the studied glasses which was the result of formation of P–O–Cu instead of P–O–P bonds. The increasing amount of copper introduced to the amorphous matrix of glasses at the cost of decreasing amount of MgO and CaO was the reason of the increasing degree of internal strains relaxation in the glass structure which was accompanied by the decrease of transformation temperature (T g) value and the increase of glass transformation parameters such as change of molar heat (∆c p) and structural relaxation time.
References
Chahine A, Et-tabirou M, Elbenaissi M, Haddad M, Pascal JL. Effect of CuO on the structure and properties of (50−x/2)Na2O–xCuO–(50−x/2)P2O5 glasses. Mater Chem Phys. 2004;84:341–7.
Shih PY, Chin TS. Effect of redox state of copper on the properties of P2O5–Na2O–CuO glasses. Mater Chem Phys. 1999;60:50–7.
Shih PY, Yung SW, Chin TS. Thermal corrosion behavior of P2O5–Na2O–CuO glasses. J Non Cryst Solids. 1998;224:143–52.
Jamnicky M, Znášik P, Tunega D, Ingram MD. Glass formation and structure in the system Cu2O–P2O5–MoO3. J Non Cryst Solids. 1995;185:151–8.
Mekki A, Holland D, McConville CF. X-ray photoelectron spectroscopy study of copper sodium silicate glass surfaces. J Non Cryst Solids. 1997;215:271–82.
Bobkova NM, Artem’eva SA, Trusova EE. Behavior of copper oxide in silicoborate glazed glasses. Coat Enamles Glass Ceram. 2007;64(7–8):264–6.
Metwalli E, Karabulut M, Sidebottom DL, Morsi MM, Brow RK. Properties and structure of copper ultraphosphate glasses. J Non Cryst Solids. 2004;344:128–34.
Lee J, Yano T, Shibata S, Yamane M. Structure and properties of ion-exchanged Na2O–Cu2O–Al2O3–SiO2 glass system. J Non Cryst Solids. 1997;222:120–4.
Lee J, Yano T, Shibata S, Nukui A, Yamane M. EXAFS study on the local environment of Cu+ ions in glasses of the Cu2O–Na2O–Al2O3–SiO2 system prepared by Cu+/Na+ ion exchange. J Non Cryst Solids. 2000;277:155–61.
Stoch L, Stoch Z, Wacławska I. Silicate glass fertilizer. Patent PL 185 229 B1. 2003 [Polish].
Krzywy E. Fertilization of soils and plants. Szczecin: University of Agriculture; 2000 [Polish].
Soliman AA, Kashif I. Copper oxide content dependence of crystallization behavior, glass forming ability, glass stability and fragility of lithium borate glasses. Phys B. 2010;405:247–53.
Agathopoulos S, Tulyaganov DU, Ventura JMG, Kannan S, Saranti A, Karakassides MA, Ferreira JMF. Structural analysis and devitrification of glasses based on the CaO–MgO–SiO2 system with B2O3, Na2O, CaF2 and P2O5 additives. J Non Cryst Solids. 2006;352:322–8.
Stoch L. Crystallochemical aspects of structure controlled processes in oxide glasses. Optica Applicata. 2008;XXXVIII(1):225–35.
Wacławska I, Szumera M. Thermal behaviour of Fe-doped silicate–phosphate glasses. J Therm Anal Calorim. 2010;101:423–7.
Szumera M, Wacławska I, Olejniczak Z. Influence of B2O3 on the structure and crystallization of soil active glasses. J Therm Anal Calorim. 2010;99:879–86.
Görlich E. The effective charges and the electronegativity. Kraków: Polish Academy of Art and Science; 1997.
Sułowska J., Wacławska I., Szumera M., Olejniczak Z. Characterization of thermally induced of crystalline phases in CuO-containing silicate–phosphate glasses. J Therm Anal Calorim. 2011. doi 10.1007/s10973-011-1988-5.
Acknowledgements
The study was supported by the Grant No. N R08 0010 06 of the Polish Ministry of Science and Higher Education.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sułowska, J., Wacławska, I. & Szumera, M. Effect of copper addition on glass transition of silicate–phosphate glasses. J Therm Anal Calorim 109, 705–710 (2012). https://doi.org/10.1007/s10973-012-2328-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2328-0