Abstract
Thermal stability of PbO was studied. Reactivity of oxides in the systems PbO–M2O3 (M = In, Fe) was investigated up to 650 °C. Using the DTA and XRD methods, parts of investigated ternary oxide systems, labelled by compounds: V2O5, Pb8V2O13 and M2O3 (M = In, Fe), have been divided into partial ternary systems. IR spectra of compounds Pb2MV3O11 (M = In, Fe) have been compared.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ternary oxide systems PbO–V2O5–M2O3 (where M = In, Fe) are worthy of study because their components as well as compounds forming in their side systems have found a lot of applications, e.g. as catalysts in different processes [1–4], as electrode materials [5] or as glasses with relatively high electrical conductivity and high thermal stability [6, 7]. It can be expected that also new phases forming with an involvement of all components of these ternary systems will have interesting properties, too.
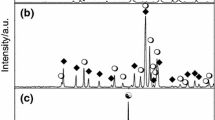
Recently, the reactivity of vanadates in two of the cross-sections of the systems PbO–V2O5–M2O3, have been investigated. As a result of the conducted reactions three new compounds were obtained [8–10], namely Pb2InV3O11 in the system InVO4–Pb2V2O7 [8], whereas Pb2FeV3O11 [9] and Pb2Fe2V4O15 [10] in the system FeVO4–Pb2V2O7. Basic physicochemical properties and crystallographic data of these new phases are known [8–10]. Both Pb2InV3O11 and Pb2FeV3O11 crystallize in the monoclinic system, but, based on XRD investigations, it was stated that they are not isostructural [8, 9]. In Fig. 1 the X-ray diffraction patterns of both compounds [8, 9] are shown for comparison. Pb2InV3O11 and Pb2FeV3O11 melt incongruently, at 800 ± 5 °C [8] and 650 ± 5 °C [9], respectively. Pb2Fe2V4O15 melts also incongruently at 640 ± 5 °C, but it crystallizes in the triclinic system [10].
The presented study was focused on determining the range of co-existence of these new phases with other compounds occurring in the systems PbO–V2O5–M2O3 (M = In, Fe) and on checking whether any other phases, unknown hitherto, are formed in these systems, involving all their components. The aim of the research has been realized by studies on the phase equilibria in the subsolidus areas of the ternary oxide systems in the whole concentration range of their components. These results are very important to study both possibility and limitation of the practical application of the obtained new multicomponent vanadates.
Experimental
The following reagents were used in the study:
-
PbO (p.a., Aldrich), V2O5 (p.a., Aldrich), In2O3 (p.a., Aldrich), α-Fe2O3 (p.a., POCh)
-
vanadates: FeVO4, Fe2V4O13, Pb2V2O7, Pb2FeV3O11, Pb2Fe2V4O15, InVO4, Pb2InV3O11 obtained by the procedures described in the papers [8–11]
-
vanadate PbV2O6, obtained as a result of heating the equimolar mixture of PbO and V2O5 in three 20-h stages at 440 °C.
Reactions were carried out by the standard method of sintering samples described inter alia in the works [12–14]. The mixtures, containing reagents weighed in appropriate proportions, were homogenized by grinding in a mechanical agate mortar and then they were heated in the atmosphere of air for several stages, with intermediate grinding. The phase composition of the samples was checked on the base of their XRD patterns, recorded after each heating stage. The phase equilibrium was stated when the composition of given samples did not change after two consecutive heating stages at temperatures several dozen of degrees lower than their melting temperatures. Melting temperatures of the samples were read in each case from their DTA curves as the onset temperature of the first recorded endothermic effect (in this study effects due to polymorphic transitions were not registered on DTA curves). Accuracy of reading the temperatures of thermal effects on DTA curves, as determined by repetitions, amounted to ± 5 °C. The results of the DTA investigations enabled to conduct reactions in the solid- state. The exact conditions of calcining samples are given in Tables 1, 2, 3, 4.
The DTA investigations were conducted in static air atmosphere using the Paulik–Paulik–Erdey type derivatograph (MOM, Hungary). Samples of 500 mg were heated in quartz or in alumina crucibles in the temperature range 20–1,000 °C with a constant heating rate of 10 °C min−1.
The XRD patterns of the samples were recorded with the aid of the diffractometer HZG4/A2 (Carl Zeiss, Germany) with the radiation CuKα/Ni. The identification of phases, present in the samples, was conducted based on their XRD characteristics contained in the PDF cards and on the data presented in the papers [8–10].
The IR spectra of the Pb2InV3O11 and Pb2FeV3O11 compounds were obtained with the use of the spectrometer Specord M 80 (Carl Zeiss, Germany), applying the technique of pressing pastilles of the investigated sample with KBr in a ratio 1:300 by mass.
Results and discussion
Thermal stability of lead(II) oxide
It is known from the literature that PbO forms two stable modifications: low-temperature tetragonal PbO-L (litharge) and high temperature orthorhombic PbO-M (massicot) [15, 16]. The temperature of the transformation PbO-L ↔ PbO-M, assessed in the study [15], is equal to 489 °C. The rate of reverse transition PbO-M ↔ PbO-L is very low at room temperature, thus, PbO-M can exist in metastable state over prolonged period [16]. Under heating in air, lead(II) oxide oxidizes to Pb3O4 which heated at higher temperatures decomposes back to PbO [15]. The temperature of the reversible reaction Pb3O4 ↔ PbO-M, assessed in the study [15], amounts to 535 °C. From the literature data it follows that the value of the melting temperature of lead(II) oxide lies between 830 and 897 °C [15], depending on the source. It is also known that lead(II) oxide sublimes before melting. This process, according to the data given in [17], was observed in temperature range 627–877 °C.
The research in the present study was begun with the verification of thermal stability of lead(II) oxide. The results of the XRD investigations have been shown that commercial PbO, used in the presented study, is the mixture of its two polymorphic modifications. DTA measurements for this oxide were conducted in alumina crucible to avoid the reaction of PbO with crucible material. On DTA curve of PbO studied only one endothermic effect was recorded (Fig. 2). Melting temperature of lead(II) oxide, read as the onset temperature of this effect, is equal to 890 ± 5 °C. The aim of the next stage of the research was to determine the maximum temperature of heating of some samples containing PbO at which sublimation of PbO is yet negligible. The samples of PbO were heated in alumina crucibles in 20-h stages at 600, 650, 700, 750 and 800 °C. The mass losses, stated after these heating stages, were: 0.21, 0.22, 0.33, 0.36 and 0.65 %, respectively. Based on the obtained results it was decided that ‘safe’ maximum temperature of heating of some samples containing PbO is about 650 °C. Such temperature enables to conduct reaction with PbO without the deviation from stoichiometry.
Phases in the side system PbO–V2O5
In the binary oxide system PbO–V2O5 five compounds, i.e. PbV2O6, Pb2V2O7, Pb3V2O8, Pb4V2O9 and Pb8V2O13 [18, 19] were reported. In order to verify the literature data, eleven mixtures of the oxides were prepared with their composition covering the whole component concentration range of the system PbO–V2O5. These samples were heated in several stages at the temperatures from the range 430–750 °C, depending on their composition. Five lead(II) vanadates(V), reported by authors [18, 19], were confirmed to form by solid-state reaction.
Study of the side systems PbO–M2O3, where M = In, Fe
In the available literature no phase diagram of PbO–In2O3 system and no information about phases forming in the reaction between the oxides PbO and In2O3 have been found. To check the reactivity of oxides in the PbO–In2O3 system five mixtures were prepared of the compositions corresponding to the PbO:In2O3 molar ratio of 3:1, 2:1, 1:1, 1:2 and 1:3. These samples were heated in the following stages: 500 °C(12 h), 600 °C(12 h), 650 °C(2 × 12 h). After the last stage of heating the diffractograms of the samples did not reveal any new diffraction lines besides those characteristic for the oxides PbO and In2O3. From the conducted experiments it can be concluded that in the system PbO–In2O3, studied only up to 650 °C, no phases are formed.
The system PbO–Fe2O3, contrary to the PbO–In2O3 system, was the object of numerous investigations. Nevertheless, there are still some disagreements about the kind of phases forming in this system. From the literature it is known that formation of the phases in the PbO–Fe2O3 system presents some problems due to a low-mutual reactivity of the oxides as well as due to PbO evaporation during thermal treatment [20, 21]. Some authors [21–23] use another methods of synthesis instead of solid- state reactions between PbO and Fe2O3. According to the latest informations [20, 24] three phases, with their compositions corresponding to: Pb2Fe2O5, Pb2Fe10O17 and PbFe12O19, are formed in the investigated system. In the presented study attempts to verify the kind of phases formed in the PbO–Fe2O3 system were undertaken. Nine mixtures were prepared of the compositions corresponding to the PbO:Fe2O3 molar ratio of 3:1, 2:1, 1:1, 1:2, 2:5, 1:3, 1:4, 1:6, 1:9. Probably, the divergences concerning the kind of phases existing in the PbO–Fe2O3 system, are caused by evaporation of PbO and as a consequence by deviation from stoichiometry. Therefore, prepared mixtures were heated in seven 20-h stages at the ‘safe’ temperature 650 °C, at which evaporation of PbO is negligible. However, XRD analysis results of these samples after their last heating stage testified that equilibrium state was not attained in this reasonable time.
Then, at this stage of research phase equilibria up to the solidus line of the systems PbO–M2O3 (M = In, Fe) have not been determined.
Subsolidus area of the system PbO–V2O5–In2O3
The investigations, intended to determine phase equilibria in the subsolidus area of the oxide system PbO–V2O5–In2O3, were begun with the study of phase equilibria in one of cross-sections of this system, i.e. PbO–InVO4. 15 mixtures of oxides were prepared for the research. The compositions of these samples, in terms of PbO and InVO4, are listed in Table 1. Table 1 presents also conditions of heating of these samples as well as the formulae of the compounds identified in the samples after their last heating stage. The obtained results imply that PbO and InVO4 do not form a real binary system, PbO–InVO4 is only an intersection of the ternary oxide system PbO–In2O3–V2O5, which crosses its (at least) six partial ternary systems. In the concentration range above 80.00 mol% of PbO (i.e. in the polygon labelled by the compounds: Pb8V2O13, PbO, In2O3) phase equilibria have been not determined.
It has been earlier established that the intersection Pb2V2O7–InVO4 of the ternary system is in the subsolidus area a real binary system in the whole concentration range of its components, in which Pb2InV3O11 undergoes crystallization [8]. On the basis of the data taken from Table 1, from the phase equilibrium diagram of the system Pb2V2O7–InVO4 [8] and from the phase diagrams of PbO–V2O5 [19] and In2O3–V2O5 [25, 26] it was possible to propose a preliminary division of the part of the subsolidus area of PbO–V2O5–In2O3 system into the regions corresponding to partial ternary systems. The division proposed did not include the polygon whose apices corresponded to the compounds: V2O5, PbV2O6, Pb2V2O7, Pb2InV3O11 and InVO4. In order to establish the phase relations in this polygon and to verify the predicted partial systems in the other area of the system PbO–V2O5–In2O3, the mixtures of oxides were prepared of the compositions chosen to represent the ranges hitherto not studied and some hypothetical partial systems comprised in the oxide systems studied. The composition of initial mixtures from which the second series samples were obtained are given in Table 2. Due to the thermal properties of PbO–V2O5 system, especially relatively low-melting temperature of PbV2O6 (about 500 °C) forming in this area, samples were heated at about 400 °C in very long cycles (over 100 h). The resulting samples were not at equilibrium state. In order to corroborate such a division of the subsolidus area of the system PbO–V2O5–In2O3, eight various mixtures were prepared from separately obtained PbV2O6, Pb2V2O7, Pb2InV3O11, InVO4 and oxides: V2O5 and In2O3. The composition of these mixtures, the stages of heating and phases detected in the equilibrium state are presented in Table 2.
On the ground of the obtained results and of the literature data [8], the part of the ternary oxide system PbO–V2O5–In2O3, labelled by compounds: V2O5, Pb8V2O13 and In2O3, has been divided into eight partial ternary systems (Fig. 3), in which there coexist in equilibrium three solid phases in each case. The same figure also shows the melting points of the mixtures of phases coexisting at equilibrium in given partial ternary system. These temperatures were read as the onset temperature of the first endothermic effect recorded in the DTA curve of given mixture. The melting points of the compounds existing in the side systems are also presented.
Subsolidus area of the system PbO–V2O5–Fe2O3
As in the case of the system PbO–V2O5–In2O3, studies on subsolidus area of the PbO–V2O5–Fe2O3 system were begun with the investigation of phase equilibria establishing in one of its cross-sections, i.e. PbO–FeVO4. 12 mixtures of oxides were prepared for the study. Table 3 presents composition of these samples in terms of PbO and FeVO4, their heating conditions and phase composition of the samples being at equilibrium state. Presented data shows that phase equilibria in the cross-section PbO–FeVO4 have been investigated only up to 80.00 mol% PbO in the initial mixtures (sample 12). On the base of the obtained results it has been stated that the cross-section PbO–FeVO4, as the cross-section PbO–InVO4, is an intersection of the ternary system of oxides, crossing its (at least) seven partial ternary systems. In the samples with PbO content above 80.00 mol% in the initial mixture, the presence of Pb2Fe2O5 was noted. Therefore, in this concentration range, the phase existing in the PbO–Fe2O3 system, is formed. Thus, in this concentration range the phase equilibria were not determined.
The presented results as well as the results of the earlier studies on the system Pb2V2O7–FeVO4 [9, 10] and literature data according to the system Fe2O3–V2O5 [11, 27], allowed a preliminary dividing of the part of the subsolidus area of the system PbO–V2O5–Fe2O3 into partial ternary systems. The proposed division did not embrace the polygon labelled by the compounds: V2O5, PbV2O6, Pb2V2O7, Pb2FeV3O11, Pb2Fe2V4O15 and FeVO4. In this area the presence of Fe2V4O13 can be expected. However, the synthesis of pure Fe2V4O13 by solid-state reaction is difficult and requires very long time of heating [11]. On the other hand, the presence of lead(II) metavanadate(V) (t m = 500 ± 5 °C), forming in this area, can hinder attainment of equilibrium state (as in the case of the system PbO–V2O5–In2O3, mentioned above). Owing to these facts, it was decided that to establish which compounds coexist at equilibrium in this polygon, ready-made phases will be used. Nine mixtures were prepared with their compositions corresponding to partial ternary systems, that can be expected based on the previous results of investigations and on the law of neighbouring phase regions. The composition of these samples (also in terms of components of the PbO–V2O5–Fe2O3 system) are given in Table 4. These samples were heated in two 20-h stages at temperatures several dozen of degrees lower than their melting temperatures. The contents of all samples did not change after these heating stages. This proves that the phases contained in the mixtures do not react with each other, but they coexist at equilibrium state.
Figure 4 presents a division of the part of the ternary oxide system PbO–V2O5–Fe2O3, labelled by compounds: V2O5, Pb8V2O13 and Fe2O3, into eleven partial ternary systems, in which there coexist in equilibrium three solid phases in each case. The melting temperatures of the mixtures of phases being at equilibrium, representing given partial ternary systems, are also shown in the Fig. 4. These temperatures were read as the onset temperature of the first endothermic effect recorded in the DTA curve of given mixture. The melting temperatures of the compounds existing in the side systems are also shown.
IR spectra of the compounds Pb2MV3O11 (M = In, Fe)
Figure 5 shows the IR spectrum of Pb2FeV3O11 (curve a) in comparison with the IR spectrum of Pb2InV3O11 (curve b). (The IR spectrum, recorded for Pb2FeV3O11, is the same as presented in the earlier work [9]). The differences in positions and intensities of absorption bands recorded in these spectra confirm the earlier assumption [8, 9] that the compounds Pb2MV3O11 (M = In, Fe), forming in analogous ternary oxide systems and having the same general formula, are not isostructural. The absorption bands recorded in the higher wave number range are sharp and intense. It suggests a deformation of the polyhedra building the structure of both compounds. Considering the fact, that the full structures of both compounds are unknown, univocal attribution of the recorded absorption bands to the concrete vibrations is not possible. However, in the light of the literature data [28–34], probable attribution can be made. The intensive absorption bands recorded in the wave number range about 1,050–630 cm−1, with a big probability, can be ascribed to stretching vibrations of V–O bonds in VO4 or/and in VO5 polyhedra, first maximum of this band being due to stretching vibrations of the shortest V–O bond. The band with its maximum recorded at 1,010 cm−1, characteristic for Pb2FeV3O11 (curve a), is shifted much more towards higher wave number than analogous band in IR spectrum of Pb2InV3O11 (curve b), which suggests higher degree of distortion of VO x polyhedra building Pb2FeV3O11 structure. In this wave number range some absorption bands connected with stretching vibrations of Fe–O bonds in FeO5 polyhedra as well as of the bridging bonds V–O–V, V–O–Fe and V–O–In cannot be excluded. In the remaining wave number range, i.e. 630–300 cm−1, much less intense absorption bands were recorded. Based on the literature data, these bands are most probably due to stretching vibrations within the MO6 (M = Fe, In) octahedra and/or the PbO x polyhedra. They may be caused by deformation vibrations of O–V–O and V–O–V bonds or they can have a mixed character.
Conclusions
PbO and MVO4 (M = In, Fe) do not form real binary systems up to the solidus line. In the ternary oxide system PbO–V2O5–In2O3 one compound, involving all its components, is formed, i.e. Pb2InV3O11. In the analogous ternary oxide system PbO–V2O5–Fe2O3 two compounds, involving all its components, are formed, i.e. Pb2FeV3O11 and Pb2Fe2V4O15. The IR spectrum of Pb2InV3O11 confirms the earlier statement that compounds Pb2MV3O11 (M = In, Fe) are not isostructural. In the parts of the ternary oxide systems: PbO–V2O5–In2O3 and PbO–V2O5–Fe2O3, labelled by compounds: V2O5, Pb8V2O13 and M2O3 (M = In, Fe), eight and eleven partial ternary systems can be distinguished, respectively. In all of them three compounds coexist at equilibrium state. In no ternary system formation of new phase, other than the known, is found.
References
Routray K, Zhou W, Kiely CJ, Wachs IE. Catalysis science of methanol oxidation over iron vanadate catalysts: nature of the catalytic active sites. ACS Catal. 2011;1:54–66.
Maji SK, Mukherjee N, Mondal A, Adhikary B. Synthesis, characterization and photocatalytic activity of α-Fe2O3 nanoparticles. Polyhedron. 2012;33:145–9.
Rakesh K, Khaire S, Bhange D, Dhanasekaran P, Deshpande SS, Awate SV, Gupta NM. Role of doping- induced photochemical and microstructural properties in the photocatalytic activity of InVO4 for splitting of water. J Mater Sci. 2011;46:5466–76.
Zakharchenko NI. Katalizatory dlya okisleniya ammiaka sistemy Fe2O3–PbO. Izv Vyssh Uchebn Zaved Khim Khim Tekhnol. 2001;44:70–5.
Zhang H, Ouyang J. High-performance inverted polymer solar cells with lead monoxide- modified indium tin oxides as the cathode. Org Electron. 2011;12:1864–71.
Saddeek YB, Shaaban ER, Aly KA, Sayed IM. Characterization of some lead vanadate glasses. J Alloys Compd. 2009;478:447–52.
Saddek YB, Shaaban ER, Aly KA, Sayed IM. Crystallization kinetics of Li2O–PbO–V2O5 glasses. Phys B. 2009;404:2412–8.
Bosacka M. New indium lead(II) vanadate(V) in Pb2V2O7–InVO4 system and its characterization. J Alloys Compd. 2012;542:228–31.
Blonska-Tabero A. A new iron lead vanadate Pb2FeV3O11: synthesis and some properties. Mater Res Bull. 2009;44:1621–5.
Blonska-Tabero A. Pb2Fe2V4O15—a new phase forming in the system FeVO4–Pb2V2O7. J Alloys Compd. 2010;508:42–6.
Walczak J, Ziolkowski J, Kurzawa M, Osten-Sacken J, Lysio M. Studies on Fe2O3–V2O5 system. Pol J Chem. 1985;59:255–62.
Bosacka M, Filipek E, Šulcova P, Dohnalová Ž, Paczesna A. Phase equilibria in the solid state and colour properties of the CuO–In2O3 system. J Therm Anal Calorim. 2012;109:605–10.
Blonska-Tabero A, Bosacka M, Dabrowska G, Filipek E, Piz M, Rychlowska-Himmel I, Tabero P, Tomaszewicz E. The synthesis and properties of the phases obtained by solid–solid reactions. J Min Metall. 2008;44B:19–26.
Filipek E, Dąbrowska G. Synthesis and selected properties of CrSbVO6 and phase relations in V2O5–Cr2O3–α-Sb2O4 system in the solid state. J Mater Sci. 2007;42:4905–15.
Risold D, Nagata J-I, Suzuki RO. Termodynamic description of the Pb-O system. J Phase Equilib. 1998;19:213–33.
Gavrichev K, Bolshakov A, Kondakov D, Khoroshilov A, Denisov S. Thermal transformations of lead oxides. J Therm Anal Calorim. 2008;92:857–63.
Lopatin SI, Mittova IY, Gerasimov FS, Shugurov SM, Kostryukov VF, Skorokhodova SM. Formation of the vapour and thermodynamic properties of the system PbO–V2O5. Zh Neorg Khim. 2006;51:1749–56.
Tsuzuki A, Kani K, Watari K, Torii Y. Preparation and properties of rapidly quenched glasses in the V2O5–PbO system. J Mater Sci. 1992;27:5091–4.
Fotiev AA, Slobodin BV, Khodos MY. Vanadates, synthesis, structure, properties. Moscow: Science; 1988.
Rivolier JL, Ferriol M, Abraham R, Cohen-Adad MT. Study of the PbO–Fe2O3 system. Eur J Solid State Inorg Chem. 1993;30:727–39.
Nevrita M, Fischer K. Contribution to the binary phase diagram of the system PbO–Fe2O3. Mater Res Bull. 1986;21:1285–90.
Yang N, Yang H, Jia J, Pang X. Formation and magnetic properties of nanosized PbFe12O19 particles synthesized by citrate precursor technique. J Alloys Compd. 2007;438:263–7.
Gil DM, Carbonio RE, Gomez MI. Synthesis of Pb2Fe2O5 by thermal decomposition of Pb2[Fe(CN)6]·4H2O. J Chil Chem Soc. 2010;55:189–92.
Diop I, David N, Fiorani JM, Podor R, Vilasi M. Experimental investigations and thermodynamic description of the PbO–Fe2O3 system. Thermochim Acta. 2010;510:202–12.
Touboul M, Melighit K. Synthesis by “chimie douce” and characterization of indium vanadates. Eur J Solid State Inorg Chem. 1994;31:151–61.
Solov’eva AE. Phase transformations in polycrystalline indium oxide. Refract Ind Ceram. 1987;28:380–5.
Doornkamp C, Clement M, Ponec V. The isotopic exchange reaction of oxygen on metal oxides. J Catal. 1999;182:390–9.
Lavat AE, Grasselli MC, Baran EJ. The IR spectra of the (Cr x Fe1−x )VO4 phases. J Solid State Chem. 1989;78:206–8.
Baran EJ, Botto IL. Crystallographic data and IR spectrum of AlVO4. Monatsh Chem. 1977;108:311–8.
Iordanova R, Dimitriev Y, Dimitrov V, Klissurski D. Structure of V2O5–MoO3–Fe2O3 glasses. J Non Cryst Solids. 1994;167:74–80.
White WB, Roy R. Infrared spectra-crystal structure correlations: II. Comparison of simple polymorphic minerals. Am Mineral. 1964;49:1670–87.
Vuk AŠ, Orel B, Dražič G, Colomban P. Vibrational spectroscopy and analytical electron microscopy studies of Fe–V–O and In–V–O thin films. Monatsh Chem. 2002;133:889–908.
Roncaglia DI, Botto IL, Baran EJ. Characterization of a low-temperature form of InVO4. J Solid State Chem. 1986;62:11–5.
Touboul M, Popot A. Synthesis, crystal chemistry and physical properties of new forms of RVO4 (R = In, Fe, Cr, Al, Nd, Y). Rev Chim Miner. 1985;22:610–24.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Blonska-Tabero, A., Bosacka, M. Comparative studies in subsolidus areas of ternary oxide systems PbO–V2O5–In2O3 and PbO–V2O5–Fe2O3 . J Therm Anal Calorim 113, 137–145 (2013). https://doi.org/10.1007/s10973-013-2996-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-2996-4