Abstract
Co-precipitation of alumina/YAG precursor from aluminum and yttrium nitrates solution with ammonium carbonate results in dawsonite (NH4Al(OH)2CO3). Its crystallographic parameters differ from the compound precipitated without the yttrium additive. It indicates that yttrium ions become incorporated into the dawsonite structure. The DSC/TG and X-ray measurements show decomposition of dawsonite at elevated temperature resulting in γ-Al2O3 which transforms to δ and θ modifications at still higher temperatures. The full transformation to α-Al2O3 and YAG occurs at temperatures higher than 1,230 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mineral dawsonite (NaAl(OH)2CO3) has been known since the nineteenth century. A variety of dawsonite-type structure compounds have been synthesized by different techniques with several incorporated cations: K+, Ca2+, Mg2+, Ba2+, Sr2+, Co2+, Mn2+ La3+, Cr3+, Fe3+ [1]. The ammonia version of dawsonite (NH4Al(OH)2CO3) was synthesized by Moringa et al. [2]. They introduced the aqueous solution of NH4Al(SO4)2 to the NH4HCO3 solution receiving the precipitate. They studied thermal decomposition of this precipitate to α-Al2O3. Ammonia dawsonite occurred as the first product of precipitation from aqueous solution of Al(NO3)3 + Mg(NO3)2 treated with (NH4)2CO3 [3]. This study was devoted to the magnesium/aluminum spinel powder synthesis; Mg/Al ratio corresponded to this one compound. After aging dawsonite of such a composition decomposed to ammonium dawsonite and also hydrotalkite (Mg6Al2(CO3)(OH)16·4H2O). X-ray amorphous ammonia version of dawsonite appeared in some studies on YAG synthesis [4–6]. Precipitation from the relative nitrate solution with NH4HCO3 was applied in these works without, however, detailed information on thermal decomposition of this compound.
The study of the alumina–YAG system by precipitation of yttria precursor within alumina suspension leads to ceramic matrix composites of interesting mechanical properties [7]. YAG inclusions resulted from the reaction between α-Al2O3 matrix and yttria during sintering.
Co-precipitation from aluminum and yttrium nitrates solution with (NH4)2CO3 allowed us to obtain an intimate mixture in the alumina–yttria system. It proved to be another possibility of the synthesis of such powders and materials [8]. This paper is devoted to characterization of the co-precipitation product and its evolution at elevated temperature. According to our knowledge, the alumina/yttria system prepared by co-precipitation with (NH4)2CO3 and its thermal evolution has not yet been investigated by other researchers.
Experimental
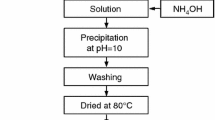
Yttria of purity 99.9 % (main impurities Cr2O3—0.04 wt% and Ag2O—0.03 wt%) was dissolved in nitric acid of analytical quality and mixed with the aluminum nitrate solution also of analytical quality. Concentration of the Al(NO3)3 + Y(NO3−)3 solution was 0.62 mol/L. The Al/Y ratio corresponded to 20 vol.% YAG (22,2 wt%) after the reaction within the system. The mutual solution of both nitrates was introduced to the vigorously stirred solution of ammonium carbonate; pH of the suspension was kept constant during the process at the level of 8.5. Under such conditions the quantitative yttrium and aluminum precipitation occurs, as determined by the ICP filtrate analysis. The surplus volume of the liquid was removed by the vacuum filtration, using Buechner funnel. The precipitate was washed three times with ethyl alcohol and then dried at 110 °C for 12 h. Washing with alcohol, due to its low surface tension, allows fluffy precipitate to be received [9]. For comparison the same procedure was applied using the Al(NO3)3 solution with no yttrium additive.
DSC/TG measurements (Netzsch STA 449 F3 Jupiter) were used to follow the phenomena occurring during heat treatment of the powder sample. The rate of temperature increase was 10 °C min−1. Measurements were performed in flowing (40 cm3 min−1) synthetic air atmosphere. Based on these measurements, the samples were prepared by their heating to the predetermined temperatures corresponding to the particular heat effects and quickly cooled down. Rate of temperature decrease from 800 to 400 °C was about 50 °C min−1. At still higher temperature it was even faster. The phase composition of the samples, heated with the DSC/TG equipment to the predetermined temperatures corresponding to the particular heat effects, was determined by the X-ray diffraction (Cu Kα radiation, equipment X’PertPro, Panalytical). Fast sample cooling allows us to assume that phase composition at predetermined temperature is freeze down. The chemical analysis of gasses emitted by the sample was useful in understanding the decomposition process of the co-precipitated product at elevated temperature. Quadrupole mass spectrometer (Mass Spectrometer QMD 300 Thermostar) was used. Helium atmosphere was applied to avoid a reaction of the emitted gasses with O2 or N2. Before measurements apparatus was washed for 24 h with He of 10 ppm H2O content. Ionic currant corresponding to this water contamination was subtracted from the analysis result.
Results and discussion
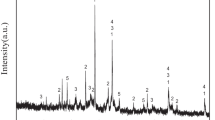
Figure 1 shows X-ray diffraction pattern of the material co-precipitated with yttrium additive. The pattern agrees with the data corresponding to ammonium dawsonite, NH4Al(OH)2CO3 (orthorhombic symmetry, space group Cmcm). The same concerns the material precipitated without yttrium additive. Identification was made on the basis of ICSD collection code 010027 and data of Ref. [10]. It is worth noticing that no reflections that could be attributed to the yttrium containing phase are observed. It suggests that yttrium ions are built into the dawsonite structure or remain in the X-ray amorphous form. However, the former eventuality seems to be corroborated by the essentially different lattice parameters of the material co-precipitated with yttrium and the one prepared by the same technique but without this additive (Table 1). The differences surpass an error of these measurements (0.0002 nm). Yttrium additive affects also the dawsonite crystallite sizes assessed on the basis of the (110) reflection broadening, using the Scherrer formula. The one with yttrium and without this additive show values of 6.1 and 12.8 nm, respectively. It indicates that yttrium ions slow down the dawsonite crystallite growth. Probably the X-ray amorphous dawsonite occurring in the study on YAG synthesis [4–6] should be related to a much higher yttrium concentration than in this study.
Figure 2 demonstrates results of the DSC/TG measurements of the material co-precipitated with yttrium (a) and containing only pure dawsonite with no yttrium additive. At low temperatures a strong endothermic effect accompanied by weight losses occurs. Weight loss in the temperature range 20–200 °C equals to 53 and 49 % for the system without yttrium and with yttrium additive, respectively. As indicated by the analysis of gasses emitted by the samples these weight losses should be attributed to the NH3, CO2, and H2O discharge (Fig. 3). At temperature higher than about 200 °C no NH3 and CO2 occurs. However, at temperature range of 200–450 °C emission of H2O is observed and it can presumably be attributed to the water desorption from the system. Weight losses in this temperature range were 9 and 8 % for the system without yttrium and with yttrium additive, respectively. The temperature of the effects differs by a few degrees centigrade for the both systems (see Fig. 2a, b). Lower peak temperature is observed in yttrium containing material. These effects result from the dawsonite decomposition as substantiated by the H2O, NH3, and CO2 emission.
Exothermic effect temperatures shown by the DSC curves (Fig. 2) of the material co-precipitated with Y additive and without this element differ essentially; peak temperature of the former one is at 946 °C and the latter, 871 °C. In order to identify the reason of these effects both samples were heated up to 800 and 1,100 °C, i.e., lower and higher than the effects under discussion. This was performed using the DSC/TG equipment. X-ray diffractions shown in Fig. 4, represent a sample containing yttrium additive. Again, no reflections which could be attributed to the yttrium containing phase could be observed neither at 800 °C nor at 1,100 °C. The material calcined at 800 °C shows γ-Al2O3 only. Heat treatment at 1,100 °C leads to alumina transformations: δ-Al2O3 and θ-Al2O3 phases appear. So we can conclude that the exothermic effect corresponds to the phase transformation: γ-Al2O3 → δ-Al2O3 + θ-Al2O3. The same is observed in the system without yttrium additive though in the case of pure alumina system temperature of this transformation is lower (cf. Fig. 2).
Crystallization of Y2O3 was observed at 800 °C during studies of YAG synthesis by co-precipitation with NH4HCO3 [4]. It cannot be excluded that an essentially higher concentration of yttrium in the case of YAG synthesis than in the present investigation shifts at least a part of yttrium component of the system towards an amorphous phase which transforms at elevated temperatures to Y2O3. It does not occur in the present investigation. It just goes again to show that yttrium builds into the dawsonite structure. That is why the first yttrium containing phases that appear at elevated temperatures are yttrium aluminates (see the further text).
The same technique as above was applied in the case of the highest temperature exothermic effect. Peak temperature in the case of yttria containing system corresponds to 1,231 °C. Figure 5 shows X-ray diffraction patterns of the sample heated up to 1,198 and 1,280 °C, e.g., slightly higher and slightly lower than the peak temperature of the exothermic effect (Fig. 2a). The former ones indicate the existence of α-Al2O3, hexagonal YAlO3 (YAH) and rhombohedra YAlO3 (YAP). The material calcined to 1,280 °C shows only α-Al2O3 and YAG (Y3Al5O12). These facts lead us to the conclusion that the high temperature exothermic effect is caused by the α-Al2O3 and yttrium aluminates crystallization. In the case of the system without yttrium additive the crystallization of α-Al2O3 is responsible for the exothermic effect (Fig. 2b). We can only speculate why peak temperature in the case of the pure system is higher than in the system with yttrium additive. Presumably transformation of YAH and YAP to YAG adds energy to the system and by itself shifts crystallization of α-Al2O3 towards lower temperatures in the system doped with yttrium. As it was found YAH and YAP transformations to YAG are exothermic ones [4, 6].
Conclusions
A mutual solution of Al(NO3)3 Y(NO3)3 was introduced into (NH4)2CO3. This procedure results in ammonium dawsonite precipitation with yttrium ions built into its structure. DSC/TG measurements at low temperature show an endothermic effect accompanied by weight loss and H2O, CO2, and NH3 emission. At the end of this process γ-Al2O3 was found by the X-ray diffraction. At 1,100 °C γ-Al2O3 transforms to δ-Al2O3 and θ-Al2O3 with no traces of yttrium containing phase. The latter appears at 1,198 °C as YAH and YAP together with α-Al2O3 and at 1,280 °C transforms to α-Al2O3 + YAG occurs.
References
Yalfani MS, Santiago M, Perez-Rairez J. In situ studies during thermal activation of dawsonite-type compounds to oxide catalysts. J Mater Chem. 2007;17:1222–9.
Moringa K, Torikai T, Nakagawa K, Jujino S. Fabrication of fine α-Al2O3 powders by thermal decomposition of ammonium aluminum carbonate hydroxide (AACH). Acta Mater. 2000;48:4735–41.
Wajler A, Tomaszewski H, Drożdż-Cieśla E, Weglarz H, Kaszkur Z. Study of magnesium spinel formation from carbonate precursors. J Eur Ceram Soc. 2008;28:2495–500.
Li J, Chen F, Liu W, Zhang W, Wang L, Ba X, Zhu Y, Pan Y, Guo J. Co-precipitation synthesis route to yttrium aluminum garnet (YAG) transparent ceramics. J Eur Ceram Soc. 2012 (in press).
Li J, Li J, Chen Q, Wu W, Xiao D, Zhu J. Effect of ammonium sulfate on the monodispersed Y3Al5O12 nanopowders synthesized by co-precipitant method. Powder Technol. 2012;212:46–50.
Li J-G, Ikegami T, Lee J-H, Mori T, Yajima Y. Co-precipitation synthesis and sintering of yttrium aluminum garnet (YAG) powders: the effect of precipitant. J Eur Ceram Soc. 2000;20:2395–405.
Lach R, Haberko K, Bućko MM, Szumera M, Grabowski G. Ceramic matrix composites in the alumina/5–30 vol.% YAG system. J Eur Ceram Soc. 2011;31:1889–95.
Lach R, Haberko K, Bućko MM. Alumina/YAG 20vol.% composite. Synthesis by the co-precipitation method. In: Proceedings of the 12th conference of the European ceramic society, Stockholm. 2011.
Haberko K. Characteristics and sintering behaviour of zirconia ultrafine powders. Ceram Int. 1979;5:145–8.
Iga T, Kato S. Crystal structure of NH4-dawsonite. J Ceram Soc Jpn. 1978;86:509–12.
Acknowledgements
Research was financially supported by the Polish Ministry of Science and High Education under Grant NN 507 457737.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lach, R., Bućko, M.M., Haberko, K. et al. Dynamic study of ammonium dawsonite doped with yttrium transformation at elevated temperatures. J Therm Anal Calorim 112, 727–730 (2013). https://doi.org/10.1007/s10973-012-2634-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2634-6