Abstract
A new compound with the formula Cu13Fe4V10O44 has been obtained as a result of a solid-state reactions occurring during heating the mixtures both of oxides: CuO, V2O5, Fe2O3 and vanadates: Cu5V2O10, Cu3Fe4V6O24. The DTA measurements were used to choose the heating temperatures as well as for determination of thermal stability of the new compound. Cu13Fe4V10O44 melts incongruently at 790 ± 5 °C. The new phase crystallizes in the monoclinic system. The indexing results and the calculated unit cell parameters for Cu13Fe4V10O44 are given. Its infra-red spectrum and image obtained by means of an electron scanning microscope are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vanadates(V) of di- and trivalent metals belong to the few catalysts which one able to stop the process of oxidation of some organic compounds at the intermediate stage, e.g. corresponding to olefins or aldehydes. It is known, for example, that copper(II) vanadates(V) are catalytically active in the reaction of oxidative dehydrogenation of isobutane to isobutene [1], whilst iron(III) orthovanadate(V) catalyses methanol oxidation to formaldehyde [2]. In view of the above, it can be expected that the phases forming as a result of reactions of the above vanadates(V) will also prove attractive from the catalytic application point of view. Thus, the system CuO–V2O5–Fe2O3 seems as a reasonable choice in the search for new potential catalysts of selective oxidation of organic compounds.
It is known that in one of the cross sections of the CuO–V2O5–Fe2O3 system, i.e. in the system FeVO4–Cu3V2O8, a double vanadate(V) of the formula Cu3Fe4V6O24 is formed [3–5]. This compound is met in two crystallographic modifications. The α-Cu3Fe4V6O24 form is a mineral called lyonsite [6]. The synthesis of Cu3Fe4V6O24 by the conventional solid-state reaction leads to its another form, i.e. β-Cu3Fe4V6O24 [3–5] whose structure is like that of mineral howardevansite, NaCuFe2V3O12 [7]. According to Belik et al. [8], the phase of the lyonsite type structure can be obtained by the conventional method of sintering, but the phase thus obtained has a composition different from that describing the mineral, and moreover, it has a certain range of homogeneity as expressed by Cu3+1.5x Fe4−x V6O24 (0.667 ≤ x < 0.778). The two minerals were discovered in the summit crater fumaroles of the Izalco volcano [6, 7]. The phases of the structures of lyonsite and howardevansite also form in other analogous ternary systems [8–11].
Belik et al. [8] studied the reactivity of oxides only for a limited range of concentrations of FeVO4–Cu3V2O8 system, i.e. from 14.29 to 50.00 mol% Cu3V2O8 in the initial mixtures. Interestingly, diffractograms of some samples containing from 30.15 to 50.00 mol% Cu3V2O8, have revealed a number of unidentified reflexes besides those characteristic for Cu2V2O7 and for lyonsite structure phase [8]. This observation could mean that besides the phases of lyonsite and howardevansite structures, there is one more phase in the ternary system CuO–V2O5–Fe2O3 in which all components are engaged. The authors [8] have not attempted to determine of the composition of this unknown phase.
The aim of the study presented was to establish the composition of the new phase forming in the CuO–V2O5–Fe2O3 system and determine its some physicochemical properties.
Experimental
Samples were obtained from the following oxides: CuO (p.a., Fluka), V2O5 (p.a., Riedel-de Haën), and α-Fe2O3 (p.a., POCh). Only one sample was prepared from vanadates: Cu5V2O10 [12, 13] and β-Cu3Fe4V6O24 [3–5]. These vanadates were obtained as a result of sintering the stoichiometric mixtures of appropriate oxides in the following stages:
-
synthesis of Cu5V2O10: 560 °C(20 h) + 750 °C(20 h) × 2
-
synthesis of β-Cu3Fe4V6O24: 560 °C(20 h) + 590 °C(20 h) + 610 °C(20 h) + 625 °C(20 h) [4]
The XRD characteristics of Cu5V2O10 and β-Cu3Fe4V6O24 obtained this way were consistent with PDF data (cards: 70-1326 and 80-0220, respectively).
The reactions, both amongst oxides as well as between Cu5V2O10 and β-Cu3Fe4V6O24, were carried out in the solid state by conventional method of sintering samples [14–17]. The initial mixtures of reactants, after homogenising by grinding, were heated in porcelain crucibles in the atmosphere of air for several stages until the state of equilibrium was attained. On completion of each heating stage, the samples were gradually cooled down in furnace to room temperature, ground and investigated by XRD method with respect to their compositions. Some of the samples were investigated by DTA method too. This procedure was repeated until the compositions of the samples did not change after two consecutive heating stages. The heating temperatures were about 60 °C below the melting points of the samples, as determined by DTA method. The melting temperatures of the samples were read as the onset temperature of the first endothermic effect recorded in the DTA curve of given sample. Accuracy of reading these temperatures (±5 °C) was determined by repetitions.
The DTA/TG investigations (the Paulik–Paulik–Erdey type derivatograph; MOM, Hungary) were performed in air atmosphere at a rate of 10 °C min−1. Samples of 500 mg were heated in quartz crucibles in the temperature range 20–1,000 °C.
Samples were studied by means of an X-ray diffractometer HZG4/A2 (Carl Zeiss, Germany). The source of radiation used was a copper tube equipped with a nickel filter. The identification of phases occurring in the samples was performed on the base of their XRD characteristics contained in the PDF cards. The powder diffraction pattern of the new compound was indexed by means of the Dicvol program [18], using α-Al2O3 as the internal standard. The parameters of the unit cell were refined by the Refinement program of DHN/PDS package.
The density of the new phase was determined using the method described in the work [19].
The IR measurement (Specord M 80; Carl Zeiss, Germany) was conducted in the wavenumber range of 1,400–250 cm−1, applying the technique of pressing pellets of the new compound with KBr in a ratio 1:300 by weight.
The investigations by SEM/EDX methods were carried out by means of an electron scanning microscope (JSM-1600, Jeol, Japan) with an X-ray energy dispersive analysis (ISIS-300, Oxford).
Results and discussion
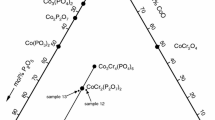
The compositions of the samples prepared for investigation, the methods of their heating, and XRD analysis results of these samples after their last heating stage are given in Table 1. The position of the investigated samples in the component concentration triangle of the CuO–V2O5–Fe2O3 system is shown in Fig. 1. The position of Cu3Fe4V6O24 [3–5] and the lyonsite structure phase [8] are also marked.
The study was started with checking if the composition of the unknown phase (hereafter X-phase) corresponds to the formula Cu2FeVO6, as according to the literature in some analogous ternary systems, the compounds of the general formula M2AVO6 are formed, e.g. Ni2FeVO6 [20], Cu2BiVO6 [21], Cd2InVO6 [22].
A mixture of the composition corresponding to the formula Cu2FeVO6 was prepared (Table 1 sample 1). The diffractogram of this sample, being in the state of equilibrium, showed a set of reflexes which could not have been assigned to any of the known phases forming in the CuO–V2O5–Fe2O3 system, besides the reflexes attesting to the presence of α-Fe2O3 and Cu5V2O10. It was assumed that this set of reflexes characterises the unknown X-phase. According to the results, the sample was not monophase, and so the composition of the new phase must be different from that described by the formula Cu2FeVO6.
In the ternary system, the composition of the forming phase corresponds often to the point of intersection of the system’s cross sections. Three mixtures were prepared for the study. Their compositions (Table 1) correspond to the intersection points of the cross section of Cu3V2O8–Fe2O3 with the systems: FeVO4–Cu5V2O10 (sample 2), Cu5V2O10–Cu3Fe4V6O24 (sample 3) and FeVO4–Cu11V6O26 (sample 4). According to the above data, the new phase is most probably formed in the Cu5V2O10–Cu3Fe4V6O24 system. The composition of sample 3 expressed in the components of the Cu5V2O10–Cu3Fe4V6O24 system corresponds to the Cu5V2O10:Cu3Fe4V6O24 ratio of 3:1. At the equilibrium state, there is an excess of Cu5V2O10 in this sample. Two other samples were prepared of the compositions corresponding to the Cu5V2O10:Cu3Fe4V6O24 ratio of 2.5:1 (sample 5) and 2:1 (sample 6). In sample 6, at the state of equilibrium neither the initial reagents nor any of the phases known to form in the CuO–V2O5–Fe2O3 were found. The diffractogram of this sample showed only the set of reflexes assumed to correspond to the unknown X-phase.
Three more samples were prepared of compositions similar to that of sample 6 (Table 1, samples 7–9). In all these samples at the state of equilibrium, the presence of X-phase along with the other phases known to form in the ternary system studied was confirmed.
Analysis of all above data permits concluding that as a result of heating a mixture of the composition 65.00% mol CuO, 25.00% mol V2O5 and 10.00% mol Fe2O3 (sample 6), a new phase has been formed whose composition can be described by the formula Cu13Fe4V10O44. Hence, the new phase is formed in the solid–state reaction according to the equation:
The new phase was also synthesised using as initial reagents the components of the system Cu5V2O10–Cu3Fe4V6O24. A mixture of the composition 66.67 mol% Cu5V2O10 and 33.33 mol% Cu3Fe4V6O24 was heated for two 20 h stages at 720 °C. The diffractogram of the sample taken after the first 20 h of heating showed only the set of reflexes characteristic for the phase Cu13Fe4V10O44. Therefore, the new phase was obtained in the solid–state reaction according to the equation:
Cu13Fe4V10O44 has a brown sort of colour, its density amounts to d obs = 3.97(5) g/cm3. The powder diffractogram of the new phase was subjected to indexing, results of which are given in Table 2. Cu13Fe4V10O44 crystallizes in the monoclinic system, its primitive unit cell parameters are as follows: a = 1.3976(2) nm, b = 1.7788(3) nm, c = 0.7810(1) nm, β = 105.65(2)°. The unit cell volume V = 1.8696 nm3; the number of stoichiometric units in the unit cell Z = 2; the XRD calculated density d = 4.02 g/cm3.
A SEM image of Cu13Fe4V10O44 (Fig. 2) shows the presence of only one of crystals’ habit. Crystals of the new compound are differentiated in size. The sizes of the larger crystals are of the order of 20 μm, whereas the sizes of the smaller crystals do not often exceed 10 μm. The results of experimental determination of composition by EDX analysis of monophase sample showed that the Cu:Fe:V ratios are near 13:4:10 (on average: 48.28 at% Cu, 13.97 at% Fe and 37.75 at% V) and correspond to the values from formula of the new compound, i.e.: 48.15 at% Cu, 14.81 at% Fe and 37.04 at% V.
Figure 3 shows the IR spectrum of the new compound. In the light of information on the literature, the absorption bands located in the wave number range of 1,050–600 cm−1 can be ascribed to the stretching vibrations of the V–O bonds in the VO4 and VO5 polyhedra [23–26]. They can be due to the stretching vibrations of the Fe–O bonds in the FeO5 and FeO4 polyhedra, too [24, 26, 28]. The absorption bands recorded in the remaining wave number, i.e. 600–300 cm−1 can be associated with stretching vibrations of the M–O bonds within FeO6 and CuO x polyhedra as well as with bending vibrations of O–V–O bonds [24–28].
The DTA curve recorded for Cu13Fe4V10O44 (Fig. 4) shows two endothermic effects beginning at temperatures close to each other: at 790 ± 5 °C and about 800 °C, respectively. To establish the type of transformation causing these effects, three samples of Cu13Fe4V10O44 compound were prepared. They were additionally heated for 2 h at different temperatures from the range 780–820 °C, and then rapidly cooled to room temperature, and then they were subjected to XRD study. The first sample was heated at 780 °C (close to the beginning of the first endothermic effect), the second at 800 °C (just after the beginning of the effect), whilst the third sample was heated at 820 °C (at the temperature corresponding to a half of the height of the first endothermic effect). On removal from the furnace, the samples heated at 800 or 820 °C were molten, whilst in the one heated at 780 °C, the liquid was not observed. The diffractogram of the sample heated at 780 °C did not differ from that of Cu13Fe4V10O44. The diffractograms of the samples heated at 800 and 820 °C were the same and did not show reflexes characteristic for Cu13Fe4V10O44. Thus, it was concluded that the endothermic effect beginning at 790 ± 5 °C is related to melting of the new compound and the process of its melting was characterised as incongruent. The only solid phase, occurring in the CuO–V2O5–Fe2O3 system, identified in the samples after their melting, was α-Fe2O3 with a melting point above 1,000 °C. As indicated by the course of the DTA curve of the new compound, it melts with separation of the second solid phase of melting point similar to that of Cu13Fe4V10O44. Because of the closeness of temperatures of these two endothermic effects in the experiment conditions (“freezing” at 780, 800 and 820 °C); no reliable assignment of the second product of melting of the new compound has been made. At this stage of the study, it can be supposed that it is Cu5V2O10 with the melting point at 805 ± 5 °C. Studies aimed at resolving this question will be continued.
Conclusions
Reaction occurring in the solid state between oxides CuO, V2O5 and Fe2O3, taken at a molar ratio 13:5:2, yielded new compound with the formula Cu13Fe4V10O44. This new phase crystallizes in the monoclinic system, and its unit cell parameters are as follows: a = 1.3976(2) nm, b = 1.7788(3) nm, c = 0.7810(1) nm, β = 105.65(2)°. Cu13Fe4V10O44 melts incongruently at 790 ± 5 °C. The new compound can be also obtained as a result of solid–state reaction between Cu5V2O10 and Cu3Fe4V6O24, taken at a molar ratio 2:1.
References
Hikazudani S, Kikutani K, Nagaoka K, Inoue T, Takita Y. Anaerobic oxidation of isobutane I. Catalysis by Cu–V complex oxides. Appl Catal A. 2008;345:65–72.
Häggblad R, Massa M, Andersson A. Stability and performance of supported Fe–V-oxide catalysts in methanol oxidation. J Catal. 2009;266:218–27.
Lafontaine MA, Grenéche JM, Laligant T, Férey G. β-Cu3Fe4(VO4)6: structural study and relationships; physical properties. J Solid State Chem. 1994;108:1–10.
Wieczorek-Ciurowa K, Rakoczy J, Blonska-Tabero A, Filipek E, Niziol J, Dulian P. Mechanochemical synthesis of double vanadate in Cu–Fe–V–O system and its physicochemical and catalytic properties. Catal Today. 2010. doi:10.1016/j.cattod.2010.12.007.
Zolnierkiewicz G, Guskos N, Typek J, Anagnostakis EA, Blonska-Tabero A, Bosacka M. Competition of magnetic interactions in M3Fe4V6O24 (M(II) = Zn, Cu, Mn, Mg) compounds studied by EPR. J Alloys Compd. 2009;471:28–32.
Hughes JM, Starkey SJ, Malinconico ML, Malinconico LL. Lyonsite, Cu 2+3 Fe4 3+(VO4) 3−6 , a new fumarolic sublimate from Izalco volcano, El Salvador: descriptive mineralogy and crystal structure. Am Mineral. 1987;72:1000–5.
Hughes JM, Drexler JW, Campana CF, Malinconico ML. Howardevansite, NaCu2+Fe2 3+(VO4) 3−3 , a new fumarolic sublimate from Izalco volcano, El Salvador: descriptive mineralogy and crystal structure. Am Mineral. 1988;73:181–6.
Belik AA, Malakho AP, Pokholok KV, Lazoryak BI. Phase formation in Cu3+1.5x R4−x (VO4)6 (R = Fe and Cr) systems: crystal structure of Cu2.5Fe4.333(VO4)6, Cu4Fe3.333(VO4)6, and Cu4.05Cr3.3(VO4)6. J Solid State Chem. 2001;56:339–48.
Kurzawa M, Bosacka M, Filipek E, Rychlowska-Himmel I. The homogeneity range of lyonsite- type phase existing in the CrVO4–Co3V2O8 system. Cent Eur J Chem. 2009;7:179–83.
Blonska-Tabero A. Phase relations in the CoO–V2O5–Fe2O3 system in subsolidus area. J Therm Anal Calorim. 2007;88:201–5.
Blonska-Tabero A. New phase in the system FeVO4–Cd4V2O9. J Therm Anal Calorim. 2008;93:707–10.
Birnie RW, Hughes JM. Stoiberite, Cu5V2O10, a new copper vanadate from Izalco volcano, El Salvador, Central America. Am Mineral. 1979;64:941–4.
Dąbrowska G, Filipek E. Reactivity of the oxides in the ternary V2O5–CuO-α–Sb2O4 system in air. J Therm Anal Calorim. 2008;93:839–45.
Šulcova P, Vitásková L, Trojan M. Thermal analysis of the Ce1−x Tb x O2 pigments. J Therm Anal Calorim. 2010;99:409–13.
Blonska-Tabero A. Pb2Fe2V4O15–a new phase forming in the system FeVO4–Pb2V2O7. J Alloys Compd. 2010;508:42–6.
Blonska-Tabero A, Bosacka M, Dabrowska G, Filipek E, Piz M, Rychlowska-Himmel I, Tabero P, Tomaszewicz E. The synthesis and properties of the phases obtained by solid–solid reactions. J Min Metall. 2008;44B:19–26.
Tabero P, Filipek E, Piz M. Reactivity of T-Nb2O5 or H-Nb2O5 towards V2O5. Synthesis in the solid state and properties of V4Nb18O55. Cent Eur J Chem. 2009;7:222–7.
Boultif A, Louër D. Indexing of powder diffraction patterns for low-symmetry lattices by the successive dichotomy method. J Appl Crystallogr. 1991;24:987–93.
Kluz Z, Waclawska I. Precise determination of powder density. Rocz Chem. 1975;49:839–41.
Kurzawa M, Blonska-Tabero A. Phase equilibria in the system NiO–V2O5–Fe2O3 in subsolidus area. J Therm Anal Calorim. 2004;77:65–73.
Radosavljevic I, Evans JO, Sleight AW. Synthesis and structure of bismuth copper vanadate, BiCu2VO6. J Solid State Chem. 1998;141:149–54.
Bosacka M. A novel compound with the formula Cd2InVO6. Mater Res Bull. 2009;44:2252–4.
Eguchi M, Komamura A, Miura T, Kisi T. Lithiation characteristics of Cu5V2O10. Electrochim Acta. 1996;41:857–61.
Baran EJ, Botto IL. Kristallographische daten und IR-spectrum von AlVO4. Monatsh Chem. 1977;108:311–8.
Zhuravlev VD, Velikodnyi YuA, Kristallov LV. Issledovanie fazovykh ravnovesii v sisteme CuO–SrO–V2O5. Zh Neorg Khim. 1987;32:3060–3.
Iordanova R, Dimitriev Y, Dimitrov V, Klissurski D. Structure of V2O5–MoO3–Fe2O3 glasses. J Non-Cryst Solids. 1994;167:74–80.
McDevitt NT, Baun WL. Infrared absorption study of metal oxides in the low frequency region (700–240 cm−1). Spectrochim Acta. 1964;20:799–808.
Selvan RK, Augustin CO, Berchmans LJ, Saraswathi R. Combustion synthesis of CuFe2O4. Mater Res Bull. 2003;38:41–54.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Blonska-Tabero, A. The synthesis and some properties of new compound Cu13Fe4V10O44 . J Therm Anal Calorim 110, 1161–1166 (2012). https://doi.org/10.1007/s10973-011-2082-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2082-8