Abstract
The purpose of this study was to determine the possibility of producing hydrophobic mesoporous mineral–carbon sorbents from aluminum hydroxide and compositions of coal tar pitch–polymers on carbonization at 600 °C in a nitrogen atmosphere. Blends of the products of co-precipitation of aluminum hydroxide in the carbonaceous substances medium were subjected to carbonization process. The extent of porous structure development was evaluated using low temperature nitrogen adsorption, adsorption of benzene vapors, and adsorption of iodine from aqueous solution. The highest value of BET surface area of about 370 m2/g was achieved for the carbonization product obtained from co-precipitated raw components with 10 wt% compositions coal tar pitch–polymer. These materials demonstrated high capacity to reduce organic pollutions from sewage. Pitch–polymer composition containing poly(ethylene terephthalate) or phenol–formaldehyde resin was studied by the means of DSC method in order to determine the high-temperature transformations taking place under the conditions of carbonization. DSC method enables to determine i.a. the decomposition temperatures of carbonizates produced from pitch–polymer compositions and the evaluation of their sorption abilities. The additive of poly(ethylene terephthalate) and phenol–formaldehyde resin caused the increase of thermal resistance of the pitch expressed by higher decomposition temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Literature references present the possibilities of use of various mineral and carbon substances to produce complex sorbents. Often used mineral substances of highly developed surface are silica and alumina while among organic substances are both chemical compounds of defined composition, like acenaphthene, naphthalene, formaldehyde, alcohols, and mixtures of organic substances, for instance olefins and industrial waste [1–7].
Chemical composition of the mixture, being the substrate for the carbonization process, and the method of mixing the components of the mixture are both important in the process of complex sorbent production. It influences the porous structure of the final product and its hydrophilic–hydrophobic properties. Because the contribution of carbonaceous component has strong influence on the structure of carbonizates, it is important to control the process of carbonaceous pyrolyzate deposition in order to obtain reproducible materials [7].
Mineral–carbon sorbents are produced by mechanical mixing of mineral and carbon substances or the deposition of carbon substance in the structure of mineral by inserting carbon precursors between gel particles and then its carbonization. They are competitive to both active carbons and mineral sorbents such as alumina. They exhibit a synergism of properties expressing itself by stronger development of mesoporous structure compared to the structure of mineral and carbon sorbents. Moreover, compared to active carbons, they have better mechanical resistance and allow to shape their hydrophobic–hydrophilic surface character [8].
A new method of synthesis and characterization of mesoporous carbonaceous materials was presented in the work [1]. It was shown possibility of synthesis mesoporous carbon by the following steps: impregnation mineral matrix by carbon precursor, carbonization, dissolving of matrix or its removing by thermal degradation. Specific surface area of these materials was about 1000 m2/g.
Authors [9] obtained microporous materials from the pitch–polymer compositions. Carbonization of these materials was carried out in two stages. Initial carbonization was conducted by heating the sample to the temperature of 520 °C with the temperature increase rate 5 °C/min in nitrogen atmosphere and holding the sample in this temperature for 1 h. The second stage of carbonization was carried out by heating the sample to the temperature 520 °C (rate 15 °C/min) and then to 850 °C (rate 5 °C/min). Activation was conducted by selective gasification with water vapor in the temperature of 800 °C until 50% mass loss was achieved. The results obtained for pitch–PET compositions indicate influence of modification coal tar pitch on development of specific surface area (424 m2/g), compared to the carbon pitch (323 m2/g).
It seems that above presented methods of sorbents preparation are more expensive from economical point of view. The purpose of this study was to determine the possibility of producing hydrophobic mesoporous mineral–carbon sorbents from aluminum hydroxide and compositions of coal tar pitch–polymers by carbonization at 600 °C in nitrogen atmosphere. Pitch–polymer composition containing poly(ethylene terephthalate) or phenol–formaldehyde resin were studied by the means of DSC method in order to determine the high-temperature transformations taking place under the conditions of carbonization. DSC method enables to determine i.a. the decomposition temperatures of carbonizates produced from pitch–polymer compositions and the evaluation of their sorption abilities. The additive of poly(ethylene terephthalate) and phenol–formaldehyde resin caused the increase of thermal resistance of the pitch expressed by higher decomposition temperatures.
Blends of the products of co-precipitation of aluminum hydroxide in the carbonaceous substances were subjected to carbonization process. The extent of porous structure development was evaluated using low temperature nitrogen adsorption, adsorption of benzene vapors. The highest value of BET surface area of about 370 m2/g was achieved for the carbonization product obtained from co-precipitated raw components with 10 wt% compositions coal tar pitch–polymer. These materials demonstrated high capacity to reduce organic pollutions from sewage.
Raw materials used and methods of sample preparation
As carriers of coal pyrolyzate, granulated coal tar pitch produced by Institute for Chemical Processing of Coal, Zabrze, Poland, pitch–polymer compositions containing up to 50 wt% of poly(ethylene terephthalate) or phenol–formaldehyde resin were used. Highly porous aluminum hydroxide was applied as a mineral matrix. The precursor for the preparation of aluminum hydroxide was aluminum chloride (purified) produced by POCh Gliwice.
In order to use modified pitches for the production of mineral–carbon sorbents, actions aimed at the preparation of products of their modification having very high softening points, high coke number, and high fluidity were undertaken. Properties of coal tar pitches modified with phenol–formaldehyde resin and poly(ethylene terephthalate), containing 50% of polymeric additive, were studied.
Compositions containing phenol–formaldehyde resin had increased softening points, compared to the granulated pitch. Softening point increased with the increased amount of additive. In the case of composition containing 50%, it was not possible to liquefy it and to measure the softening point. Coke number of compositions containing granulated pitch and phenol–formaldehyde resin did not depend on the amount of polymer and was similar to the value obtained for the granulated pitch.
Poly(ethylene terephthalate) provided high increase of softening point compared to unmodified pitch. Increase of poly(ethylene terephthalate) amount added to the pitch resulted in higher softening point. For the composition containing 50% of PET the softening point was ca. 130 °C.
Addition of poly(ethylene terephthalate) to the pitch caused significant changes in group composition. The amount of compounds insoluble in toluene and quinoline increased.
The value of coking number of pitch modified with poly(ethylene terephthalate) depended on the amount of modifier. With the increase of polymer amount in compositions prepared in preservative conditions, the coking number of composition decreased, which was caused primarily by low coking value of PET [10].
Samples of mineral–carbon sorbents were prepared by the earlier elaborated method of co-precipitation of mineral matrix in the environment of organic compounds [8]. Given amount of pitch (or pitch–polymer composition) in adequate mass ratio with aluminum hydroxide was dissolved in 200 cm3 of N-methylpyrollidone. Such mixture was placed in a thermostat heated at 100 °C, and 0.5 M aluminum chloride and 1.5 M ammonia aqueous solutions were dosed into the mixture at constant rate. After finishing the dosing the mixture was annealed at 100 °C for 1 h, after which it was cooled to room temperature and separated on paper filter. Obtained precipitate was dried in a laboratory drier at 100 °C and then it was subjected to carbonization.
All samples were carbonized in nitrogen atmosphere in an electric tube furnace in 600 °C for 3 h.
Experimental methods
Thermal properties were studied using Netzsch Maia F3 scanning differential calorimeter. DSC studies allowed to examine the behavior of pitch and pitch–polymer compositions in temperatures from −30 °C to 530 °C, with the heating ratio of 3°/min. The measurements were carried out in the following cycles: heating, cooling, heating. Obtained DSC curves allowed determination of the phase transition temperatures: glass transition temperature, melting temperature, the onset of crystallization temperature, and the onset of decomposition temperature.
Measurement parameters were established on the basis of preliminary studies on coal tar pitch and polymeric modifiers:
-
for compositions containing poly(ethylene terephthalate) (crystalline polymer) the measurements were carried out during heating of the sample to a temperature higher by 30–40 °C than the expected melting temperature of the polymer, then cooling it to the temperature lower than its glass transition point and then heating to 530 °C.
-
for compositions containing an amorphous polymer, i.e., phenol–formaldehyde resin, the measurements were carried out by heating the sample to 30 °C, cooling to −30 °C and then heating to 530 °C.
Phase transition temperatures were determined as:
-
glass transition temperature—the point located in the middle of the line connecting base lines, co called “midpoint” [11],
-
crystallization temperature of the mixture during its cooling—the temperature corresponding to the extrapolated beginning of an adequate DSC curve peak, while during heating of the system–temperature corresponding to the extrapolated ending of an adequate peak on the DSC curve,
-
melting temperature—temperature related to the peak of endothermic peak of DSC curve.
-
decomposition temperature—temperature corresponding to the extrapolated beginning of an adequate peak on DSC curve.
In order to evaluate the structural properties of the samples of mineral matrix and mineral–carbon sorbents, adsorption studies were carried out in a vacuum glass apparatus. Measurements of nitrogen adsorption at −196 °C by volumetric method and adsorption of benzene vapors at 20 °C by McBain-Bakr gravimetric method were carried out. Samples were outgases at 100 °C (heating rate 1 °C/min). With the use of “Izotermy” computer software the calculations of specific surface by BET method (S BET) and mesoporous surface were conducted [12].
The IR spectra were recorded with a FTIR Matson Spectrophotometer in the wave number range of 4000–400 cm−1. Powders were dispersed in KBr matrix and pressed into thin, transparent pellets.
Adsorptive properties of obtained pitch–aluminum sorbents were tested in the process of purification of petrochemical waste waters. Waste water samples originating both from refinery and petrochemical processes were contaminated mainly with organic compounds. These studies were aimed at the evaluation of sorptive properties of mineral–carbon carbonizates for organic compounds occurring in high concentration (chemical oxygen demand values from several hundreds to several thousands mgO2/dm3).
For the raw and processed waste waters, chemical oxygen demand (COD) and ether extract were determined. Experiments of waste water purification were conducted with the use of varied amounts of all sorbents. Waste waters (1 dm3) were poured into beakers equipped with mixers and 0.5 or 1.0 g of selected sorbent was added. One of the beakers contained only raw waste water. The mixtures were mixed for 20 min (quickly for 5 min and slowly for 15 min). After sedimentation, the precipitate was separated from the solution by decantation, and above mentioned measurements were conducted.
Results
Thermal properties
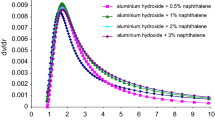
In Tables 1–3, characteristic temperatures of phase transitions determined on the basis of DSC curves of coal tar pitch modified with polymers are presented. In Figs. 1 and 2, DSC curves of pitch–poly(ethylene terephthalate) and pitch–phenol–formaldehyde resin compositions are shown.
Comparing the temperatures of the transition of the pitch from the brittle state to the viscoelastic one, it can be found that the addition of poly(ethylene terephthalate) caused the increase of the temperature of this transition. In the case of composition containing phenol–formaldehyde resin, this transition was not observed.
Comparing the melting temperatures of the pitch it was found that the addition of poly(ethylene terephthalate) and phenol–formaldehyde resin to the pitch caused slight increase in its melting temperature (by ca 2.6–6.4 °C).
Decomposition temperatures of all compositions with the addition of poly(ethylene terephthalate) and phenol–formaldehyde resin were similar and higher than the decomposition temperature of a pure pitch.
Addition of 50% of polymers caused the temperatures of decomposition of these compositions to be similar to the temperatures recorded for polymers (Table 3).
Biggest changes of phase transition temperatures occurred under the influence of poly(ethylene terephthalate) and phenol–formaldehyde resin. These polymers caused the increase of thermal resistance of the composition:
-
in the case of composition with phenol–formaldehyde resin the increase of decomposition temperature was by 50–60 °C,
-
for the compositions containing poly(ethylene terephthalate) the increase of decomposition temperature was higher and depended strongly on the amount of PET.
The high decomposition temperature (387 °C) was recorded for the composition containing 50% of PET. This additive also caused the increase of the temperature of pitch transition from brittle to viscoelastic state.
Increase of the amount of polymeric additive to coal tar pitch caused the appearance of phase transitions characteristic for polymeric modifiers. The course of DSC curves obtained for compositions containing 50% of polymer was similar to the course of DSC curves recorded for polymers.
It was found that the best modifiers of the coal tar pitch, in the aspect of their use as raw materials for the production of sorbents, because of their miscibility with pitch, high softening point, significant amount of components insoluble in toluene, high resistance to strain and high thermal resistance, can be compositions containing phenol–formaldehyde resin and poly(ethylene terephthalate). These compositions were used as a precursor of carbon pyrolyzate in pitch–aluminum sorbents.
Adsorption and structural properties
In the IR spectra recorded for example sample containing initial 10% pitch–phenol–formaldehyde resin (Fig. 3) there is a broad band at the wave number range of 3700–2900 cm−1 ascribed to stretching vibrations of OH groups in water physically adsorbed. Except the above mentioned band in the IR spectra there is another one such band at 1640 cm−1 ascribed to bending vibrations of OH groups in structural water. The process of calcination led to creation of γ-Al2O3. It is confirmed by a strong broad absorption band in the region 750–900 cm−1, due to stretching vibrations of a lattice of interlinked tetrahedra AlO4.
Specific surface of aluminum hydroxide determined on the basis of low-temperature nitrogen adsorption falls into the range of 170–180 m2/g, while the surface of aluminum oxide obtained during calcination in 600 °C is ca. 200 m2/g (Fig. 4). The development of specific surface is caused by dehydration of aluminum hydroxide. Dehydration of mineral matrix during carbonization of mineral–carbon mixture is also beneficial for the development of the final product’s surface.
Specific surface area for pitch–aluminum sorbents obtained by the method of co-precipitation with the use of unmodified coal-tar pitch. 1 Initial aluminum hydroxide, 2 aluminum hydroxide after calcination in 600 °C/3 h, 3–10 sorbents obtained by co-precipitation with the initial concentration of coal-tar pitch of: 0.5, 1, 5, 8, 10, 12, 20 and 22% mass
Low temperature nitrogen adsorption studies of the samples obtained by co-precipitation of aluminum hydroxide in the environment of coal tar pitch and carbonization indicate the increase of surface for the concentration of pitch from 0.5 to 10% in the initial mixture. The highest value of specific surface—ca. 370 m2/g—was for the sample containing 8% of pitch and samples containing 10% of pitch–poly(ethylene terephthalate) or pitch–phenol–formaldehyde resin compositions (Figs. 4, 5). It has to be noted that specific surface of obtained sorbents is significantly higher compared to the initial mineral matrix calcinated in the same conditions, which proves that the obtained product has new, different porous structure. Further increase of pitch content in the initial mixture results in the overtaking of the structure building role by carbon pyrolyzate, which results in the reduction of specific surface. Obtained results of structure development in the case of the use of pitch–polymer compositions as well as unmodified pitch are comparable, which can indicate that there is a possibility to use waste basestocks interchangeably for the production of sorbents. Reduction of coking number, as well as increase of melting temperature, decomposition temperature, and the temperature of the transition from brittle to viscoelastic state of initial pitch–polymer compositions compared to coal tar pitch does not influence the sorption ability of obtained mineral–carbon sorbents.
Specific surface S BET for pitch–aluminum sorbents obtained by the method of co-precipitation with the use of pitch–polymer compositions. 1–8 Sorbents obtained by co-precipitation with the initial concentration of 50% pitch + 50% poly(ethylene terephthalate) of: 0.5, 1, 5, 8, 10, 12, 15 and 20% mass, 9–16 sorbents obtained by co-precipitation with the initial concentration of 50% pitch + 50% phenol–formaldehyde resin of: 0.5, 1, 5, 8, 10, 12, 15 and 20% mass
Adsorption isotherms of benzene vapors recorded in order to characterize the porous structure of aluminum hydroxide and obtained mineral–carbon sorbents are presented in Fig. 6, while specific surface values are shown in Fig. 7.
Parameters of porous structure of pitch–aluminum sorbents calculated on the basis of adsorption isotherms of benzene vapors and nitrogen adsorption. 1 Aluminum hydroxide after calcination in 600 °C/3 h, 2 sorbent obtained by co-precipitation with the initial content of pitch of 8% mass, 3 sorbent obtained by co-precipitation with the initial content of 50% pitch–50% PET composition of 10% mass, 4 sorbent obtained by co-precipitation with the initial content of 50% pitch–50% PF composition of 10% mass
It was concluded that all tested samples of pitch–aluminum sorbents prepared with the use of pitch–poly(ethylene terephthalate) and pitch–phenol–formaldehyde resin compositions, as well as aluminum hydroxide, had wide hysteresis loops. It indicates that they have well-developed porous structure, where mesopores of irregular shapes are dominant.
Hysteresis loops of adsorption and desorption of benzene vapors can be qualified according to the IUPAC nomenclature to the H2 type. It corresponds to pores comparable to “inkwell” and spherical pores with open end and significant internal contractions.
In the case of sorbent samples containing initially bigger amounts of introduced pitch–polymer compositions, the decrease of sorption abilities of benzene vapors are observed, which is demonstrated by adsorption isotherms presented below. The structure-building role is overtaken in this cane by carbon pyrolyzate, having low specific surface values.
Based on calculated values of S BET specific surface with the assumption of horizontal orientation of benzene it was found that with increasing content of carbon pitch compared to mineral matrix, specific surface of obtained sorbents increases (Fig. 7). The samples containing initially 8% of coal tar pitch and 10% of pitch–polymer compositions had the highest value of S BET specific surface.
Mesopore surface values (Fig. 8) indicate that the mesopore surface (S mez) of carbonizates is higher compared to unmodified matrix. Obtained samples have different structural properties than initial matrix and carbon pyrolyzate obtained during carbonization.
Mesopore surface values of selected pitch–aluminum calculated on the basis of benzene vapors adsorption isotherms. 1 Aluminum hydroxide after calcination in 600 °C/3 h, 2, 3 sorbent obtained by co-precipitation with the initial content of 50% pitch–50% PET composition of 8 and 10% mass, 4, 5 sorbent obtained by co-precipitation with the initial content of 50% pitch–50% PF composition of 8 and 10% mass
Studies on waste-water purification with the use of selected pitch–polymer sorbents
The results of studies carried out for selected sorbents of improved effectiveness of impurities reduction are presented in Tables 4 and 5. The impurity reduction factors (in %) were calculated compared to the initial sample of (raw) waste water.
Sorbents of relatively high specific surface, homogeneously covered with carbon pyrolyzate have good sorption abilities of organic compounds. Significant reduction of substances extractable by petroleum ether (up to 81%) and COD (up to 65%) was observed, depending on the amount of used sorbents and their adsorptive properties. In all cases better purification coefficients for ether extract than for COD were obtained. It can indicate that obtained materials have good affinity to aromatic compounds. Aromatic hydrocarbons not having side alkyl chains are not oxidized during chemical oxygen demand measurements and that is why this method has significant limitations for determination of these hydrocarbons in waste-water samples.
In the case of pitch–aluminum sorbents, their particular advantage in the process of purification of waste waters contaminated with organic substances seems to be their high specific surface, mesoporous structure and hydrophobic properties determined the affinity of the surface to such adsorbates.
Summary
It was found that the best modifiers to the coal tar pitch in the aspect of using them as basestocks for the production of sorbents, because of their good miscibility with the pitch, high softening point, significant content of components insoluble in toluene, high resistance to strain and high thermal resistance, can be the compositions containing phenol–formaldehyde resin and poly(ethylene terephthalate).
The method of co-precipitation of a mineral matrix in an environment of coal tar pitch causes the incorporation of pitch particles into crystalline structure of aluminum gel and proved to be the best to prepare, during the process of carbonization, a sorbent of much more developed mesoporous structure, compared to sorbents obtained from mineral or carbon substances. Distinct synergism of interaction between mineral and organic component is well visible in this case. The process of infiltration of organic substance particles into porous structure of formed gel particles assures the covering of matrix surface with thin, homogeneous layer of carbon pyrolyzate. Carbonizates prepared by this method with the use of 10% of pitch–poly(ethylene terephthalate) or pitch–phenol–formaldehyde resin compositions have the highest values of specific surface (ca. 370 m2/g) and well-developed mesoporous structure.
Mesoporous mineral–carbon sorbents prepared by the co-precipitation method have hydrophobic surface, different from the initial hydrophilic mineral matrix. Such materials can be applied in the sorption processes of aromatic hydrocarbons from petrochemical waste waters.
Modification of coal tar pitch with polymers causes the increase of its coking number, increase of softening point, increase of decomposition temperature, does not influence significantly the porous structure of mineral–pitch sorbents, which indicates the possibility to utilize plastic wastes in the production of such materials.
References
Choma J, Jaroniec M, Zawiślak A. Mesoporous carbons: synthesis and properties. Wiadomości Chemiczne. 2008;61:373–402.
Chuah GK, Zhan X, Xu P, Joenicke S. J Mol Catal A. 2008;181:25.
Xiubin H, Zhanbin H. Zeolite application for enhancing water infiltration and retention in loess soil. Resour Conserv Recycl. 2001;34:45.
Tabak A. Structural analysis of reactive dye species retained by the basic alumina surface. J Therm Anal Calorim. 2009;95:31–6.
Błachnio M, Staszczuk P, Grodzicka G. Adsorption and porosity properties of pure and modified carbon nanotube surfaces. J Therm Anal Calorim. 2008;94:641–8.
Dweck J. Qualitative and quantitative characterization of Brazilian natural and organophilic clays by thermal analysis. J Therm Anal Calorim. 2008;92:129–35.
Szychowski D., Obtained and investigation of physicochemical properties of mineral-carbon sorbents. Doctoral Thesis, University of Lodz; 2003.
Szychowski D, Pacewska B, Zieliński J, Ciesińska W, Brzozowska T. Investigation possibility of application coal tar pitch as component of mineral-carbon sorbents. Karbo. 2009;54:56–60.
Ciesińska W, Makomaski G, Zieliński J, Brzozowska T, Pacewska B, Szychowski D. Studies on the preparation of microporous material from the pitch-polymer compositions. Pol J Environ Stud. 2009;18:27–30.
Makomaski G, Ciesińska W, Zieliński J, Brzozowska T, Pacewska B, Szychowski D. Bitum-polymer compositions as precursors of carbon sorbents. In: Pittsburgh coal conference, coal—energy, environment and sustainable development, 20–23 wrzesień 2009, Pittsburgh, USA, s. 64.
Pielichowski J. Thermal analysis methods. In: investigations of polymers. II School of Thermal Analysis SAT’98: Poland, Zakopane; 1998. pp. 131–146.
Pacewska B, Szychowski D, Żmijewski T. Forum Chemiczne 2000. Warsaw: Warsaw University of Technology; 2000.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Szychowski, D., Pacewska, B., Makomaski, G. et al. Adsorption and DSC study of mineral–carbon sorbents obtained from coal tar pitch–polymer compositions. J Therm Anal Calorim 107, 893–900 (2012). https://doi.org/10.1007/s10973-010-1257-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1257-z