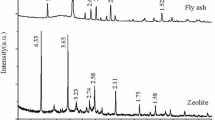
Abstract
Coal fly ash, as a solid waste produced from coal-fired power plants, was recycled for synthesis of zeolite A and geopolymer which were used for stabilization/solidification of Cs+ and Sr2+ from aqueous solutions. Specifically, the sorption data was successfully fitted by kinetic and thermodynamic models. The microstructure changes of zeolite A after loading Cs+ and Sr2+ were explored using XRD, FTIR, Raman, TG-DTA, and N2 adsorption/desorption isotherm. The solidification of the spent zeolites using geopolymer was conducted and evaluated. It was found that pseudo-second sorption mechanism was predominant and, according to the Boyd equation, film diffusion seemed to govern the sorption process. The maximum sorption capacities on Cs+ and Sr2+ based on Langmuir model were 2.12 and 1.93 mmol/g, respectively. During ion exchange with Cs+ and Sr2+, Cs+ was inclined to go through the window to occupy the position of eight-member ring, while the Sr2+ was more likely to replace the Na+ in the six-member ring, thereby easily leading to the different changes of zeolite structure. In addition, geopolymer could be a promising matrix for the treatment of radioactive waste because the leaching fraction greatly decreased after solidification by geopolymer. Therefore, the recycling of coal fly ash for radioactive waste disposal could achieve the concept of disposal waste with waste and recycling, which could greatly contribute to the sustainable development of society.












Similar content being viewed by others
References
Alby D, Charnay C, Heran M, Prelot B, Zajac J (2018) Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: synthesis and shaping, sorption capacity, mechanisms, and selectivity-a review. J Hazard Mater 344:511–530
Aronne A, Esposito S, Ferone C, Pansini M, Pernice P (2002) FTIR study of the thermal transformation of barium-exchanged zeolite a to celsian. J Mater Chem 12:3039–3045
Banerjee D, Sandhya U, Pahan S, Joseph A, Ananthanarayanan A, Shah JG (2017) Removal of Cs-137 and Sr-90 from low-level radioactive effluents by hexacyanoferrate loaded synthetic 4A type zeolite. J Radioanal Nucl Chem 311:893–902
Bhavornthanayod C, Rungrojchaipon P (2009) Synthesis of Zeolite A Membrane from Rice Husk Ash. JMMM 19:79–83
Boca Santa RAA, Soares C, Riella HG (2016) Geopolymers with a high percentage of bottom ash for solidification/immobilization of different toxic metals. J Hazard Mater 318:145–153
Busca G (2017) Acidity and basicity of zeolites: a fundamental approach. Microporous Mesoporous Mater 254:3–16
Castaldi P, Santona L, Enzo S, Melis P (2008) Sorption processes and XRD analysis of a natural zeolite exchanged with Pb2+, Cd2+ and Zn2+ cations. J Hazard Mater 156:428–434
Chaves T, Soares F, Cardoso D, Carneiro R (2015) Monitoring of the crystallization of zeolite LTA using Raman and chemometric tools. Analyst 140:854–859
de Peña YP, Rondón W (2013) Linde type a zeolite and type Y faujasite as a solid-phase for lead, cadmium, nickel and cobalt preconcentration and determination using a flow injection system coupled to flame atomic absorption spectrometry. Am J Anal Chem 4:387–397
Depla A, Verheyen E, Veyfeyken A, Gobechiya E, Hartmann T, Schaefer R, Martens JA, Kirschhock CE (2011) Zeolites X and a crystallization compared by simultaneous UV/VIS-Raman and X-ray diffraction. PCCP 13:13730–13737
Duxson P, Fernández-Jiménez A, Provis JL, Lukey GC, Palomo A, van Deventer JS (2007) Geopolymer technology: the current state of the art. J Mater Sci 42:2917–2933
El-Kamash AM (2008) Evaluation of zeolite a for the sorptive removal of Cs+ and Sr2+ ions from aqueous solutions using batch and fixed bed column operations. J Hazard Mater 151:432–445
El-Naggar MR, Amin M (2018) Impact of alkali cations on properties of metakaolin and metakaolin/slag geopolymers: microstructures in relation to sorption of Cs-134 radionuclide. J Hazard Mater 344:913–924
EPA U (1992) Method 1311. Toxicity characteristic leaching procedure. EPA SW-846: test methods for evaluating solid waste. Phys/Chem Methods
Evans L (1954) Bubble-mineral attachment in flotation. Ind Eng Chem 46:2420–2424
Fang XH, Fang F, Lu CH, Zheng L (2017) Removal of Cs+, Sr2+, and Co2+ ions from the mixture of organics and suspended solids aqueous solutions by zeolites. Nucl Eng Technol 49:556–561
Ho Y-S (2006) Second-order kinetic model for the sorption of cadmium onto tree fern: a comparison of linear and non-linear methods. Water Res 40:119–125
Ibrahim HA, Moamen OAA, Monem NA, Ismail IM (2018) Assessment of kinetic and isotherm models for competitive sorption of Cs+ and Sr2+ from binary metal solution onto nanosized zeolite. Chem Eng Commun 205:1274–1287
Izidoro JD, Fungaro DA, Abbott JE, Wang SB (2013) Synthesis of zeolites X and a from fly ashes for cadmium and zinc removal from aqueous solutions in single and binary ion systems. Fuel 103:827–834
Kaushik VK, Vijayalakshmi RP, Choudary NV, Bhat SGT (2002) XPS studies on cation exchanged zeolite a. Microporous Mesoporous Mater 51:139–144
Kemper W (1960) Water and ion movement in thin films as influenced by the electrostatic charge and diffuse layer of cations associated with clay mineral surfaces 1. Soil Sci Soc Am J 24:10–16
Koohsaryan E, Anbia M (2016) Nanosized and hierarchical zeolites: a short review. Chin J Catal 37:447–467
Król M, Mozgawa W, Barczyk K, Bajda T, Kozanecki M (2013) Changes in the vibrational spectra of zeolites due to sorption of heavy metal cations. J Appl Spectrosc 80:644–650
Kuenzel C, Cisneros JF, Neville TP, Vandeperre LJ, Simons SJR, Bensted J, Cheeseman CR (2015) Encapsulation of Cs/Sr contaminated clinoptilolite in geopolymers produced from metakaolin. J Nucl Mater 466:94–99
Lin S-L, Lai JS, Chian ES (1995) Modifications of sulfur polymer cement (SPC) stabilization and solidification (S/S) process. Waste Manag 15:441–447
Munthali M, Johan E, Aono H, Matsue N (2015) Cs+ and Sr2+ adsorption selectivity of zeolites in relation to radioactive decontamination. J Asian Ceramic Soc 3:245–250
Nikolić V, Komljenović M, Džunuzović N, Ivanovic T (2018) The influence of mechanical activation of Fly ash on the toxic metals immobilization by Fly ash-based Geopolymers, Key Engineering Materials. Trans Tech Publ 761:3–6
Noroozi R, Al-Musawi TJ, Kazemian H, Kalhori EM, Zarrabi M (2018) Removal of cyanide using surface-modified Linde type-a zeolite nanoparticles as an efficient and eco-friendly material. J Water Process Eng 21:44–51
Ogawa K, Nitta M, Aomura K (1979) A theoretical study of the site selectivity of the zeolite cation. 2. Site selectivities of alkaline earth metal cations in dehydrated fully ion-exchanged zeolite a. J Phys Chem 83:1235–1236
Palomo A (2017) Geopolymer matrices for the immobilisation of toxic wastes. Vitrification and Geopolimerization of wastes for immobilization or recycling 21
Pluth JJ, Smith JV (1982) Crystal structure of dehydrated strontium-exchanged zeolite a. absence of near-zero-coordinate strontium (2+) ion. Presence of aluminum complex. J Am Chem Soc 104:6977–6982
Reichenberg D (1953) Properties of ion-exchange resins in relation to their structure. III. Kinetics of exchange. J Am Chem Soc 75:589–597
Sanders MJ, Catlow CRA, Smith JV (1984) Crystal energy calculations from strontium ions in zeolite a. J Phys Chem 88:2796–2797
Smirnov KS, Le Maire M, Brémard C, Bougeard D (1994) Vibrational spectra of cation-exchanged zeolite a. experimental and molecular dynamics study. Chem Phys 179:445–454
Song JC, Liu MY, Zhang Y (2015) Ion-exchange adsorption of calcium ions from water and geothermal water with modified zeolite a. AIChE J 61:640–654
Tian Q, Guo B, Nakama S, Sasaki K (2018) Distributions and leaching behaviors of toxic elements in Fly ash. ACS Omega 3:13055–13064
Tian Q, Guo B, Nakama S, Zhang L, Hu Z, Sasaki K (2019) Reduction of undesirable element leaching from fly ash by adding hydroxylated calcined dolomite. Waste Manag 86:23–35
Trussell S, Spence R (1994) A review of solidification/stabilization interferences. Waste Manag 14:507–519
Vipin AK, Ling S, Fugetsu B (2016) Removal of Cs+ and Sr2+ from water using MWCNT reinforced zeolite-a beads. Microporous Mesoporous Mater 224:84–88
Xu Z, Jiang Z, Wu D, Peng X, Xu Y, Li N, Qi Y, Li P (2017) Immobilization of strontium-loaded zeolite a by metakaolin based-geopolymer. Ceram Int 43:4434–4439
Yao ZT, Ji XS, Sarker PK, Tang JH, Ge LQ, Xia MS, Xi YQ (2015) A comprehensive review on the applications of coal fly ash. Earth-Sci Rev 141:105–121
Yoshida K, Toyoura K, Matsunaga K, Nakahira A, Kurata H, Ikuhara YH, Sasaki Y (2013) Atomic sites and stability of Cs+ captured within zeolitic nanocavities. Sci Rep 3:2457
Funding
This research was supported to KS by the JSPS (Japan Society for the Promotion of Science) Kaken Kiban A project (No. JP16H02435) and to QT by the China Scholarship Council (No. 201706420068).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 103 kb)
Rights and permissions
About this article
Cite this article
Tian, Q., Sasaki, K. Application of fly ash-based materials for stabilization/solidification of cesium and strontium. Environ Sci Pollut Res 26, 23542–23554 (2019). https://doi.org/10.1007/s11356-019-05612-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05612-1