Abstract
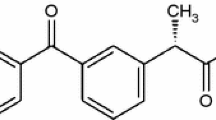
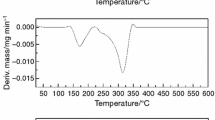
Simultaneous thermoanalytical techniques were used for the characterization of the thermal decomposition of ketoprofen—active substance and tablets. DTA and DSC curves showed that ketoprofen melts before the decomposition. A kinetic study regarding the ketoprofen—active substance’s thermal decomposition was performed under non-isothermal conditions and in a nitrogen atmosphere at five heating rates: 2.5, 5, 7.5, 10 and 15 °C min−1. The kinetic parameters of thermal decomposition process were obtained from TG/DTG curves using the following differential methods: Friedman isoconversional, Chang, respectively, integral methods: Flynn–Wall–Ozawa, Kissinger–Akahira–Sunose, Coats–Redfern and Madhusudanan. The careful treatment of the kinetic parameters obtained in certain thermal conditions was confirmed to be necessary as well as a different strategy of experimental data processing.







Similar content being viewed by others
References
Dutta S, Padhye S, McKee V. Structural characterization and SOD activity of copper-oxaprozinate. Inorg Chem Commun. 2004;7:1071–4.
Fini A, Fasio G, Benetti L, Ghedini V. Thermal analysis of some diclofenac salts with alkyl and alkylhydroxy amines. Thermochim Acta. 2007;464:65–74.
Barbato F, Cappello B, La Rotonda MI, Miro A, Quaglia F. Diclofenac/β-cyclodextrin binary systems: a study in solution and in the solid state. J Incl Phenom Macrocycl Chem. 2003;46:179–85.
Ying YC, Yi L, Cheng ZJ, Dan Z. Inhibitory effect of copper complex of indomethacin on bacteria studied by microcalorimetry. Biol Trace Elem Res. 2008;122:82–8.
Kafarska K, Czakis-Sulikowska D, Wolf WM. Novel Co(II) and Cd(II) complexes with non-steroidal anti-inflammatory drugs. Synthesis, properties and thermal investigation. J Therm Anal Calorim. 2009;96:617–21.
Bernardi LS, Oliveira PR, Murakami FS, Silva MAS, Borgmann SHM, Cardoso SG. Characterization of venlafoxine with pharmaceutical excipients. J Therm Anal Calorim. 2009;97:729–33.
Moyano MA, Broussalis AM, Segall AI. Thermal analysis of lipoic acid and evaluation of the compatibility with excipients. J Therm Anal Calorim. 2010. doi: 10.1007/s10973-009-0351-6.
Moura EA, Correia LP, Pinto MF, Procopio JVV, de Sousa FS, Macedo RO. Thermal characterization of the solid state and raw material fluconazole by thermal analysis and pyrolysis coupled to GC/MS. J Therm Anal Calorim. 2010. doi: 10.1007/s10973-009-0473-X.
Oliveira PR, Bernardi LS, Murakami FS, Mendes C, Silva MAS. Thermal characterization and compatibility studies of norfloxacin for development of extended release tablets. J Therm Anal Calorim. 2009;97:741–5.
Bannach G, Cervini P, Cavalheiro ETG, Ionashiro M. Using thermal and spectroscopic data to investigate the thermal behaviour of drugs and excipients by unique calculations. J Therm Anal Calorim. 2010. doi: 10.007/s10973-009-0595-1.
Avula SG, Alexander K, Riga A. Predicting eutectic behavior of drugs and excipients by unique calculations. J Therm Anal Calorim. 2010;99:655–8.
Salvio Neto H, Barros PAF, de Souza Carvalho MF, Matos RJ. Thermal analysis of prednicarbate and characterization of thermal decomposition product. J Therm Anal Calorim. 2010;102:277–83.
Felix SF, da Silva CCL, Angnes L, Matos RJ. Thermal behaviour study and decomposition kinetics of salbutamol under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2009;95:877–80.
Macedo OR, Aragao SFC, do Nascimento GT, Macedo CMA. Application of thermogravimetry in the quality control of chloramphenicol tablets. J Therm Anal Calorim. 1999;56:1323–7.
Macedo OR, de Souza GA, Macedo CMA. Application of thermogravimetry in the quality control of mebendazole. J Therm Anal Calorim. 1997;49:937–41.
Nunes SR, Semaan SF, Riga TA, Cavalheiro GTE. Thermal behavior of verapamil hydrochloride and its association with excipients. J Therm Anal Calorim. 2009;97:349–53.
Freire DF, Aragao SFC, de Lima AFT, Raffin NFM. Compatibility study between chlorpropamide and excipients in their physical mixtures. J Therm Anal Calorim. 2009;97:355–7.
Mura P, Gratteri P, Faucci TM. Compatibility studies of multicomponent tablet formulations. DSC and experimental mixture design. J Therm Anal Calorim. 2002;68:541–51.
Oliveira GGG, Ferraz GH, Matos RSJ. Thermoanalytical study of glibenclamide and excipients. J Therm Anal Calorim. 2005;79:267–70.
Ortega A. A simple and precise linear integral method for isoconversional data. Thermochim Acta. 2008;474:81–6.
Chrissafis K. Kinetics of thermal degradation of polymers. Complementary use of isoconversional and model-fitting methods. J Therm Anal Calorim. 2009;95:273–83.
Saha B, Maiti AK, Ghoshal AK. Model-free method for isothermal and non-isothermal decomposition kinetics analysis of PET sample. Thermochim Acta. 2006;444:46–52.
Dickinson CF, Heal GR. A review of the ICTAC kinetics project, 2000: Part 1. Isothermal results. Thermochim Acta. 2009;494:1–14.
Dickinson CF, Heal GR. A review of the ICTAC kinetics project, 2000: Part 2. Non-isothermal results. Thermochim Acta. 2009;494:15–25.
Budrugeac P. Differential non-linear isoconversional procedure for evaluating the activation energy of non-isothermal reactions. J Therm Anal Calorim. 2002;68:131–9.
Tiţa B, Marian E, Tiţa D, Vlase G, Doca N, Vlase T. Comparative kinetic study of decomposition of some diazepine derivatives under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2008;94:447–52.
Tiţa B, Fuliaş A, Marian E, Tiţa D. Thermal behaviour of acetylsalicylic acid—active substance and tablets. Kinetic study under non-isothermal conditions. Rev Chim (Bucureşti). 2009;60:419–23.
Tiţa B, Fuliaş A, Marian E, Tiţa D. Thermal stability and decomposition kinetics under non-isothermal conditions of sodium diclofenac. Rev Chim (Bucureşti). 2009;60:524–8.
Fuliaş A, Tiţa B, Bandur G, Tiţa D. Thermal decomposition of some benzodiazepines under non-isothermal conditions. Kinetic study. Rev Chim (Bucureşti). 2009;60:1079–83.
Tiţa B, Fuliaş A, Rusu G, Tiţa D. Thermal behaviour of indomethacin—active substance and tablets kinetic study under non-isothermal conditions. Rev Chim (Bucureşti). 2009;60:1210–5.
Li X, Wu Y, Gu D, Gan F. Thermal decomposition kinetics of nickel (II) and cobalt (II) azo barbituric acid complex. Thermochim Acta. 2009;493:85–9.
Giron D, Goldbronn C. Use of DSC and TG for identification and quantification of the dosage form. J Therm Anal Calorim. 1997;48:473–83.
Howell BA. Utility of kinetic analysis in the determination of reaction mechanism. J Therm Anal Calorim. 2006;85:165–7.
Tomassetti M, Catalani A, Rossi V, Vecchio S. Thermal analysis study of the interactions between acetaminophen and excipients in solid dosage forms and in some binary mixtures. J Pharm Biomed Anal. 2005;37:949–55.
Marini A, Berbenni V, Pegoretti M, Bruni G, Cofrancesco P, Sinistri C. Drug-excipient compatibility studies by physico-chemical techniques; The case of Atenolol. J Therm Anal Calorim. 2003;73:547–61.
Corvi Mora P, Cirri M, Mura P. Differential scanning calorimetry as a screening technique in compatibility studies of DHEA extended release formulations. J Pharm Biomed Anal. 2006;42:3–10.
Barboza F, Vecchia DD, Tagliari MP, Silva MAS, Stulzer HK. Differential scanning calorimetry as a screening technique in compatibility studies of acyclovir extended release formulations. Pharm Chem J. 2009;43:363–8.
Desai R, Shaikh MM, Dharwadkar SR. Preformulation compatibility studies of etamsylate and fluconazole drugs with lactose by DSC. J Therm Anal Calorim. 2003;71:651–8.
Balestrieri F, Magrì AD, Magrì AL, Marini D, Sacchini A. Application of differential scanning calorimetry to the study of drug-excipient compatibility. Thermochim Acta. 1996;285:337–45.
Genieva SD, Vlaev LT, Atanassov AN. Study of the thermooxidative degradation kinetics of poly (tetrafluoroethene) using iso-conversional calculation procedure. J Therm Anal Calorim. 2010;99:551–61.
Madhusudanan PM, Krishnan K, Ninan KN. New equations for kinetic analysis of non-isothermal reactions. Thermochim Acta. 1993;221:13–21.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiţa, D., Fuliaş, A. & Tiţa, B. Thermal stability of ketoprofen—active substance and tablets. J Therm Anal Calorim 105, 501–508 (2011). https://doi.org/10.1007/s10973-010-1187-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1187-9