Abstract
Pyridine-2,5-dicarboxylic acid, known as isocinchomeric acid is one of six isomers containing two carboxylic groups. Light lanthanide (III) complexes with pyridine-2,5-dicarboxylic acid with general formula Ln2L3·nH2O, where n = 8, 9, were obtained. Their thermal and spectroscopic properties were studied. Sodium salt was obtained as Na2L·H2O. Hydrated complexes of La(III), Ce(III), Pr(III), Nd(III), Sm(III), Eu(III) and Gd(III) are stable to 313–333 K, whereas Na2L·H2O is stable to about 333 K. Dehydration process for all compounds runs in one stage, next they decompose into appropriate lanthanide oxalates, oxocarbonates carbonates and finally to metal oxides. Bands of νCOOH vibrations at 1736 and 1728 cm−1 disappear on complex spectra and νas and νs of COO− groups appear thus indicating that complexation process took place.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid development of coordination polymers synthesis has been reported in recent years [1–3]. It is mainly caused by their potential applications and possibility of creating multidimensional structures required in biochemistry as well as in pharmacy. Due to the presence of various coordinating atoms like O and N pyridinedicarboxylic acids are ligands which play an instant role in building such crystal architectures. Large number of transition and lanthanide complexes with series of the pyridinedicarboxylic acids (PDCA), which include the 2,3-, 2,4-, 2,5-, 2,6-, 3,4- and 3,5-pyridinedicarboxylic isomers, have been reported in the recent two decades. Although there are many publications about their complexes with transition metals, there are few papers about compounds formed by 2,5-pydcH2 ligand. There is a wide range of coordination possibilities among isomers of isocinchomeric acid. They can create helicates and other supramolecular structures as well as nanostructures [4]. Isocinchomeric acid has beneficial structure to cage materials synthesis. It is mainly caused by the presence of two carboxylate groups separated by the nitrogen and carbon atom from the ring. Metal ion can be therefore easily coordinated and supramolecular and multidimentional structures can be created [5, 6]. Isocinchomeric acid can be herein promising ligand for MOFs structure building or cage materials where small molecules may be captured. Both the carboxylic groups can stabilize three-dimensional structures, and hence hydrogen bonds creation. It was reported that each carboxylic group can accept up to four hydrogen bonds [5, 6].
Hence due to the presence of five donor atoms in the molecule, pyridine-2,5-dicarboxylate anion has many different possibilities of coordination with lanthanide ions. Like Lewis base, it can also act as chelating or multidentate bridging ligand [7]. There were many compounds reported in which M–O and M–N coordination was observed [8–10]. Copper(II) ion in complex with 2,4-pyridinedicarboxylate anion was coordinated through nitrogen atom from the aromatic ring and one carboxylate anion situated next to the heteroatom, while the second carboxylic group became idle [11]. Similar coordination was observed for copper(II) complex with 3,4-pyridinedicarboxylic acid [12], zinc(II), nickel(II) [13], and cobalt(II) [14] complexes with 2,4-pyridinedicarboxylic acid. In mixed complexes, for example Ln(III)–Cu(II), transition metal ion is coordinated through nitrogen atom from the aromatic ring and carboxylate anion situated in second position, while the lanthanide ion is coordinated by the second carboxylate group (in fourth position) [15]. Therefore, it is highly possible that transition metal ions would be coordinated only by one carboxylate group and nitrogen atom, while lanthanide ions only through carboxylate anions in pyridinedicarboxylic acids. The presence of lone electron pair in the nitrogen atom from the aromatic ring and oxygen atoms from two different carboxylic groups cause the possibility of creating polymeric [16], dimeric [17] and rarely monomeric [18] structures. Lanthanides act as hard Pearson’s acids; therefore, coordination through oxygen atoms from carboxylate groups mainly takes place. There are few examples where coordination between lanthanide cation and nitrogen atom from pyridine ring takes place. Geometric orientation of donor atoms in neodymium (III) complex with 2,6-pyridinedicarboxylic acid almost force lanthanide–nitrogen bonding [19]. It is mainly caused by the symmetrically spacing of carboxylic groups in 2,6-pyridinedicarboxylic acids. The chance of such lanthanide complexation among the other isomers of pyridinedicarboxylic acid, as well as isocinchomeric acid, is far lower, mainly due to steric conditions, although we can not exclude such possibility [20]. The lanthanide (III) coordination takes place through carboxylate groups, which are deprotonated in all complexes. Hence to the high density on oxygen atoms of these groups [21], there are many ways of metal ions coordination. Carboxylate group can have chelate [22], bridging, chelate-bridging [23] or monodentate. The geometry of these groups is often different what can be the reason of different ways of metal coordination [24]. Hence, many different spacial structures can be created. The best way to define the structure of organometallic compound is to obtain a monocrystal. We did not obtain the right crystal for analysis, though we concentrated on the IR spectra of obtained complexes analysis. IR spectra analyses were presented in this article and many possibilities of lanthanide ions coordination through donor atoms were discussed.
Although knowledge about biological meaning of pyridinedicarboxylates is still very poor, 2,5-pyridinedicarboxylic acid inhibits GA 2β-hydroxylase and proline 4-hydroxylase enzymes [25]. It also accelerates the redox reactions between alcohols and chromic acid [26]. Pyridinedicarboxylic acids also act as chelating agents of chromium, zinc, manganese, copper, iron and molybdenum [7].
In this article, we report the synthesis, spectroscopic and thermal studies of obtained light lanthanide (III) complexes with isocinchomeric acid.
Experimental
Materials and methods
All chemicals employed were commercially available from Sigma and Aldrich Company and used without further purification. The purity of used acid was 98%. The IR spectra were recorded with 1725× Perkin Elmer spectrometer at the range of 4000–200 cm−1 using the KBr technique.
Thermogravimetric analyses were conducted on Setsys 16/18 analyzer in dynamic air atmosphere. Therefore, 6.96–7.51 mg samples were heated in the range of 303–1273 K in ceramic crucibles using a heating rate of 283 K min−1. TG, DTG and DTA curves were registered.
Elementary analysis was made to determine composition and formulas of the obtained complexes. The analysis was carrying out using a CHN 2400 Perkin Elmer Analyzer. The water content was determined from the thermogravimetric curves of their mass loss and elementary analysis. The metal content was determined from TG curves.
Raman spectra were registered by Renishaw Raman Microscope via Reflex with 10 min exposure time and 785 nm laser edge.
Infrared and Raman theoretical calculations in DFTB3LYP/6-311++G** were collected.
Sample preparation
All compounds were obtained in double exchange method. To the hot solution of 0.1 M lanthanide chloride (pH ca. 5.3), stochiometric amount of 0.1 M ammonium salt (pH ca. 5.8) of isocinchomeric acid was added dropwise, pH of final mixtures was about 5.0–5.3. Mixtures were heated on the electric stirrer for about 40 min at 338 K until full precipitation. Next, precipitates were filtered, washed with water to remove ammonium ions and dried at room temperature in desiccators to the stable mass. Cerium (III) complex was prepared from hexahydrated nitrate(V) with final pH 3.9.
Sodium salt was prepared from 1 M solution of sodium hydroxide into which 10% underflow of stoichiometric amount of 0.5 M 2,5-pyridinedicarboxylic acid solution was added dropwise. Final pH was equal to 7. The mixture was left on heater to remove excess of solvent. After 1 hour, it was left at room temperature for slower solvent evaporation.
Obtained complexes of lantanum, europium and gadolinium were white, samarium was light cream, neodymium was light violet, praseodymium light green and cerium light yellow. Sodium salt was white.
Results and discussion
Elemental analysis
Carbon, hydrogen and nitrogen percentage contents determined for the obtained complexes are in agreement with found values (Table 1).
Thermal analysis
Compounds obtained through the syntheses of light lanthanide ions with isocinchomeric acid were all hydrated with the general formula: Ln2L3·nH2O where n is equal to 8 or 9, depending on the particular lanthanide complex.
The metal:ligand ratio in each complex is equal 2:3. Elementary analysis confirmed the ratio.
2,5-pyridinedicarboxylic acid is thermally stable up to 523 K when one stage decomposition process takes place (found mass loss: 99,091).
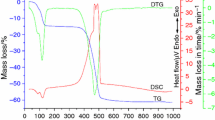
Monohydrated sodium salt was stable until 333 K when it decomposed into anhydrous complex Na2L, which is stable up to 750 K. At 763 K, further decomposition takes place with sodium carbonate as the final decomposition product. Lanthanum complex was obtained as nonahydrated compound stable at 323 K (Fig. 1). All water molecules were lost in one stage dehydration. Anhydrous lanthanide (III) pyridinedicarboxylate is stable until 693 K, when it decomposes to oxalate, at 768 K to carbonate and at 873 to La2O3. Cerium (III) and praseodymium (III) complexes, both containing nine water molecules, decomposed similarly through anhydrous compounds to appropriate metal oxides CeO2 and Pr6O11. Both complexes started their dehydration at 313 K, although anhydrous praseodymium complex seem to be more stable with temperatures of decomposition at 713 K for Pr and 648 K for Ce. Octahydrated neodymium (III) complex, as well as the rest of obtained lanthanide complexes, was stable to 333 K. Dehydration process ran in one stage. Anhydrous compound was stable until 723 K and decomposed through carbonate, oxocarbonate to Nd2O3. Thermal decomposition of Sm2L3·8H2O, Eu2L3·8H2O, Gd2L3·8H2O (Fig. 2) ran similarly through three stages for each compound. First dehydration process which began at 313 K for Sm and at 323 K for Eu and Gd. Anhydrous complexes are stable until 718 K (Sm), 673 K (Eu) and 723 K (Gd) when decomposition to oxocarbonates takes place. Calculated and found mass losses were presented in Table 2.
IR and Raman analysis
Hence to the difficulties with ascribing particular frequencies to bands on infrared spectra, theoretical IR and Raman calculations for acid were registered. These difficulties were mostly connected with bands corresponding to the ring vibrations region, which contains stretching vibrations of C=N bands from pyridine ring. According to the reported literature data, this band occurs at about 1290 and 1120 cm−1 [27] and at 3490–3450 cm−1 [28]. Hence to the theoretical data, we can assume that there are no single specific frequencies for C=N vibrations, because they are always connected with stretching or bending vibrations of pyridine ring. Stretching vibrations of C–H and C=N bands occur at 1637 and 1608 cm−1 on theoretical IR spectrum. At the range of highest frequencies, stretching vibrations of OH groups from carboxylic group of fifth and second position, respectively, are present. Frequencies at 3198, 3199 and 3216 cm−1 correspond to the stretching vibrations of hydrogen atoms C–H from aromatic ring. Bands at 1828 and 1797 cm−1 correspond to the νasCOO vibrations in second and fifth position, respectively. We can therefore assume that higher frequency value for COO group in second position is caused by some interaction between carboxylic groups placed nearest nitrogen atom from aromatic ring. At lower frequencies, different ring vibrations, breathing and bending, are present. Experimental IR spectrum of 2,5-pyridinedicarboxylic acid was interpreted on the basis of theoretical spectrum. At the range of highest frequencies, weak bands of hydroxyl group stretching vibrations νOH are present as well as quite strong bands of hydrogen vibrations from aromatic ring. At about 1736 cm−1, strong splitted band of carboxylic groups is present. At the range of 1624–1536 cm−1, there are three medium bands of aromatic ring stretching vibrations, which also appear at 1408 and 1384 cm−1. At the range of lower frequencies (1328–360 cm−1), numerous bands of ring vibrations are present with the most characteristic at 1328 cm−1 (β C–H)ar, 1012 cm−1 (γC-H in 3 and 4 position), 932 cm−1 (γC-H in 6 position) and 896 cm−1 corresponding to the breathing ring vibrations. On theoretical IR spectrum of sodium salt (Fig. 3), two bands of high intensity can be observed at about 1400 and 1580 cm−1. These bands correspond to symmetric and asymmetric vibrations of deprotonated carboxylic groups, respectively. At the range of lower frequencies, there were observed five more bands corresponding to the different ring vibrations. Frequencies for acid spectrum and all obtained light lanthanide (III) complexes spectra were collected in Table 3.
Experimental IR spectra of sodium salt (Fig. 4) and light lanthanide complexes (Figs. 5, 6) with isocinchomeric acid are very similar. At the range of highest frequencies, bands of stretching νOH group from water molecules, vibrations are present. On each complexes spectra, we can observe strong bands at about 1600 cm−1, which correspond to the stretching asymmetric vibrations of carboxylate groups. Bands of νsymCOO− vibrations occur at 1408, 1364 cm−1 (sodium salt), 1396, 1364 cm−1 (La), 1400, 1364 cm−1 (Ce, Pr), 1400, 1364 cm−1 (Nd, Eu, Gd), 1408, 1364 cm−1 (Sm). Only bands of europium (III) and gadolinium (III) complexes are little splitted. There is no bands characteristic for carboxylic group from the acid at about 1700 cm−1, what indicates that coordination processes took place in each compound. We can therefore assume that different ways of metal coordination may take place. At the range of 1288–640 cm−1, various bands of stretching, bending, wagging and out of plane ring vibrations are present on all complexes spectra. Medium intensity bands characteristic for breathing ring vibrations occur at 832 cm−1 (Na), 840 cm−1 (La, Pr), 836 cm−1 (Ce), 848 cm−1 (Nd, Sm, Eu) and 852 cm−1 (Gd). Also, medium intensity bands corresponding to metal–ligand coordination appear at the range of lowest frequencies, i.e., 508 cm−1 (Na), 512 cm−1 (La, Ce), 516 cm−1 (Pr) and 524 cm−1 (Nd, Sm, Eu, Gd).
Raman Spectra were registered for all obtained complexes. Experimental spectra of sodium, lanthanum and gadolinium complexes were presented in Figs. 7, 8 and 9, respectively. The most intensive bands are present at about 1597, 880, and 1440 cm−1 which correspond to stretching ring vibrations (νC–H, νC=N, νC–C) and bending β C–O vibrations, respectively. They are much more intensive than bands present on IR spectra. Breathing ring vibrations with medium intensity bands occur at 830 cm−1.
Conclusions
Light lanthanide (III) and sodium complexes with 2,5-pyridinedicarboxylic acid have been synthesized by the double exchange method. Based on IR and Raman analysis, possible ways of coordination have been investigated and discussed. Coordination probably takes place through carboxylate groups from 2,5-pyridinedicarboxylic ring, although we cannot exclude the possibility of coordination through nitrogen atom. Theoretical IR calculations made for isocinchomeric acid indicate on the interaction between nitrogen atom and carboxylic group placed in the second position.
Light lanthanides complexes with isocinchomeric acid are very good potential materials for large area microporous compounds synthesis. These hydrated compounds are thermally stable up to 313–333 K, and during heated process, they lose all water molecules in one stage with creation of compounds with strictly defined composition. Anhydrous complexes are thermally stable up to 673–763 K. Thermal stability of compound structure is necessary condition for their potential application as microporous compounds.
This stage of our work allowed us to precisely identify the stability and spectroscopic properties of the obtained complexes. Examination of these complexes will be continuing in the direction of obtaining microporous materials and studies on their sorption properties. Our further work will be connected with MOF-like structure synthesis in hydrothermal conditions.
References
Iwan M. Influence of preparation conditions on thermal properties of lanthanide benzenepolycarboxylates. J Therm Anal Calorim. 2007;88:157–62.
Mojumdar SC, Šimon P, Krutošíková A.. [1]Benzofuro[3,2- c]pyridine synthesis and coordination reactions. J Therm Anal Calorim. 2009;96:103–9.
Köse DA. Bis(N, N-diethylnicotinamide)p-chlorobenzoate complexes of Ni(II), Zn(II) and Cd(II). J Therm Anal Calorim. 2009;95:247–51.
Lessmann JJ. Supramolecular coordination chemistry in aqueous solution: lanthanide ion-induced triple helix formation. Inorg Chem. 2000;39:3114–24.
MacDonald JC. Preparation and thermal decomposition study of pyridinedicarboxylate intercalated layered double hydroxides. J Am Chem Soc. 2000;122:11692–702.
Gorbitz CH. Hydrogen bonds to carboxylate groups. Syn/anti distributions and steric effects. J Am Chem Soc. 1992;114:627–31.
Kita E, Marai H. Synthesis and kinetic studies in aqueous solution on chromium(III) complexes with isocinchomeronic acid—potential new biochromium sources. Transit Met Chem. 2008;33:211–7.
Xiong R-G, Xue X. Novel, acentric metal-organic coordination polymers from hydrothermal reactions involving in situ ligand synthesis. Angew Chem Int Ed. 2002;41:3800–3.
Sengupta P, Dinda R. Synthesis and characterisation of some ruthenium(II) complexes of α-N heterocyclic carboxylic acids—X-ray structures of cis-[Ru(PPh3)2(L1)2]·2CH3OH and cis-[Ru(PPh3)2(L3H)2] (L1H = pyridine 2-carboxylic acid and L3H2 = imidazole 4,5-dicarboxylic acid). Polyhedron. 2001;20:3349–54.
Wei M, Xu XY, He J. Preparation and thermal decomposition study of pyridinedicarboxylate intercalated layered double hydroxides. J Therm Anal Calorim. 2006;85:795–800.
Min D, Yoon SS, Jung D-Y. One-dimensional copper–pyridinedicarboxylate polymer containing square-planar Cu(II) centers exhibiting antiferromagnetic coupling. Inorg Chim Acta. 2001;324:293–9.
Yan S, Li X, Zheng X. Effect of the carboxyl groups on the assembly of copper pyridinedicarboxylate complexes. J Mol Struct. 2009;929:105–11.
Sileo EE, Araujo AS. Solid state coordination chemistry of pyridinedicarboxylic acid isomers. III. Synthesis and crystal structures of complexes of Zn and Ni with lutidinic acid (lutidinic = 2,4-pyridinedicarboxylic). J Mol Struct. 2003;644:67–76.
Mendoza-Diaz G, Rigotti G. Solid-state 111Cd NMR studies on cadmium(II)-2,x-pyridinedicarboxylates. Crystal structure of 2,4-pyridinedicarboxylato triaqua cadmium(II) hemihydrate: [Cd(II)(2,4-pydc)(H2O)3]·1/2H2O. Polyhedron. 2005;24:777–83.
Liang Y, Cao R. Syntheses and characterizations of two novel Ln(III)–Cu(II) coordination polymers constructed by pyridine-2,4-dicarboxylate ligand. Inoorg Chem Comm. 2002;5:366–8.
Rzączyńska Z, Bartyzel A, Olszewska E. Synthesis and characterization of Co(II), Cu(II) and Zn(II) complexes with 1,1-cyclobutanedicarboxylic acid. Polyhedron. 2006;25:687–94.
Rzączyńska Z, Belsky VK. Crystal structure of dimer tetraaquabis(μ-4-aminobenzoato-O,O′)tetrakis(4-aminobenzoato-O,O)diytterbium(III) dihydrate. Polish J Chem. 1994;68:309–19.
Rzączyńska Z, Belsky VK. Crystal structure of triaquatris(3,5-diaminobenzoato-O,O)samarium(III). Polish J Chem. 1994;68:369–75.
Yue Q, Yang J. Three-dimensional 3d–4f heterometallic coordination polymers: synthesis, structures, and magnetic properties. Inorg Chem. 2005;44:5241–6.
Rzączyńska Z, Brzyska W, Mrożek R. The crystal structures of two neodymium(III) complexes with pyridine-2,6-dicarboxylic acid. J Coord Chem. 1998;43:321–34.
Sienkiewicz-Gromiuk J, Widma doświadczalne i teoretyczne (IR, Ramana) kwasu 4,4’-bifenylodokarboksylowego. Nauka i Przemysł, UMCS University, Lublin, Poland; 2009. pp. 446–9.
Łyszczek R, Mazur L, Rzączyńska Z. A three-dimensional coordination polymer constructed from sodium(I) ion and benzene-1,2,4-tricarboxylate ligand: thermal, structure and spectroscopic characteristics. Inorg Chem Commun. 2008;11:1091–3.
Rzączyńska Z, Bartyzel A, Głowiak T. The crystal structure of a terbium(III) complex with 1,1-cyclobutanedicarboxylic acid. J Coord Chem. 2003;56:1525–30.
Kula A, Mazur L. Crystal structure, spectroscopic and thermal studies of anhydrous potassium 2,6-dihydroxybenzoate. J Coord Chem. 2008;61:1751–8.
Grigss DL. Inhibition of gibberellins 2β-hydoxylases by acylcyclohexanedione derivatives. Photochemistry. 1991;30:2513–7.
Dunn GE, Lee GKJ. Kinetics and mechanism of decarboxylation of some pyridinecarboxylic acids in aqueous solution. Can J Chem. 1972;50:3017–27.
Brzyska W, Ożga W. Preparation, properties and thermal decomposition of Y(III) and lanthanide(III) pyridine-2,5-dicarboxylates. Thermochim Acta. 1996;288:113–21.
Wasylina L, Kucharska E. The13C NMR, UV and IR absorption spectra of pyridinedicarboxylic acids. Chem Heterocycl Compd. 1999;35:186–94.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Rzączyńska, Z., Danczowska-Burdon, A. & Sienkiewicz-Gromiuk, J. Thermal and spectroscopic properties of light lanthanides (III) and sodium complexes of 2,5-pyridinedicarboxylic acid. J Therm Anal Calorim 101, 671–677 (2010). https://doi.org/10.1007/s10973-010-0941-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0941-3












