Abstract
In several current important problems in different areas of soft matter physics, controversy persists in interpreting the molecular dynamics observed by various spectroscopies including dielectric relaxation, light scattering, nuclear magnetic resonance, and neutron scattering. Outstanding examples include: (1) relaxation of water in aqueous mixtures, in molecular sieves and silica-gel nanopores, and in hydration shell of proteins; and (2) dynamics of each component in binary miscible polymer blends, in mixtures of an amorphous polymer with a small molecular glassformer, and in binary mixtures of two small molecular glassformers. We show the applications of calorimetry to these problems have enhanced our understanding of the dynamics and eliminated the controversies.



Similar content being viewed by others
Notes
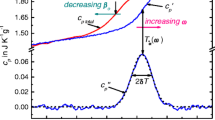
We are not interested in the peaks of enthalpy release and absorption rates observed at 210 K of water confined in 1.8 nm diameter pores of MCM-41, and is attributed to glass transition of bulk water by Oguni et al. [18].
References
Capaccioli S, Ngai KL, Shinyashiki N. The Johari-Goldstein β-relaxation of water. J Phys Chem B. 2007;111:8197–209.
Mierzwa M, Pawlus S, Paluch M, Kaminska E, Ngai KL. Correlation between primary and secondary Johari–Goldstein relaxations in supercooled liquids: invariance to changes in thermodynamic conditions. J Chem Phys. 2008;128:044512.
Kessairi K, Capaccioli S, Prevosto D, Lucchesi M, Sharifi S, Rolla PA. Interdependence of primary and Johari-Goldstein secondary relaxations in glass-forming systems. J Phys Chem B. 2008;112:4470–3.
Capaccioli S, Prevosto D, Lucchesi M, Rolla PA, Casalini R, Ngai KL. Identifying the genuine Johari-Goldstein β-relaxation by cooling, compressing, and aging small molecular glass-formers. J Non-Cryst Solids. 2005;351:2643–51.
Capaccioli S, Kessairi K, Prevosto D, Lucchesi M, Rolla PA. Correlation of structural, Johari-Goldstein relaxations in systems vitrifying along isobaric, isothermal paths. J Phys Condens Matter. 2007;19:205133.
Ngai KL. An extended coupling model description of the evolution of dynamics with time in supercooled liquids and ionic conductors. J Phys Condens Matter. 2003;15:S1107–25.
Ngai KL, Paluch M. Classification of secondary relaxation in glass-formers based on dynamic properties. J Chem Phys. 2004;120:857.
Jansson H, Swenson J. Dynamics of water in molecular sieves by dielectric spectroscopy. Eur Phys J E. 2003;12:S51–4.
Bergman R, Swenson J. Dynamics of supercooled water in confined geometry. Nature. 2000;403:283–6.
Swenson J, Jansson H, Howells WS, Longeville S. Dynamics of water in a molecular sieve by quasielastic neutron scattering. J Chem Phys. 2005;122:084505.
Hedström J, Swenson J, Bergman R, Jansson H, Kittaka S. Does confined water exhibit a fragile-to-strong transition? Eur Phys J. Special Topics. 2007;141:53–6.
Faraone A, Liu L, Mou C-Y, Yen C-W, Chen S-H. Fragile-to-strong liquid transition in deeply supercooled confined water. J Chem Phys. 2004;121:10843–6.
Liu L, Chen S-H, Faraone A, Yen C-W, Mou C-Y. Pressure dependence of fragile-to-strong transition and a possible second critical point in supercooled confined water. Phys Rev Lett. 2005;95:117802.
Mallamace F, Broccio M, Corsaro C, Farone A, Wanderlingh U, Liu L, et al. The fragile-to-strong dynamic crossover transition in confined water: nuclear magnetic resonance results. J Chem Phys. 2006;124:161102.
Swenson J. Comment on “Pressure dependence of fragile-to-strong transition and a possible second critical point in supercooled confined water. Phys Rev Lett. 2006;97:189801.
Oguni M, Maruyama S, Wakabayashi K, Nagoe A. Glass transitions of ordinary and heavy water within silica-gel nanopores. Chem Asian J. 2007;2:514–20.
Debenedetti PG. Supercooled and glassy water. J Phys Condens Matter. 2003;15:R1669–726.
Oguni M, Kanke Y, Namba S. Thermal properties of the water confined within nanopores of silica MCM-41. AIP Conf Proc. 2008;982:34.
Cammarata M, Levantino M, Cupane A, Longo A, Martorana A, Bruni F. Structure and dynamics of water confined in silica hydrogels: X-ray scattering and dielectric spectroscopy studies. Eur Phys J E. 2003;12:S63–6.
Pathmanathan K, Johari GP. Dielectric and conductivity relaxations in Poly(hema) and of water in its hydrogel. J Polym Sci B. 1990;28:675–89.
Pathmanathan K, Johari GP. Relaxation and crystallization of water in a hydrogel. J Chem Soc Faraday Trans. 1994;90:1143.
Hofer K, Mayer E, Johari GP. Glass liquid transition of water and ethylene-glycol solution in poly(2-hydroxyethyl methacrylate) hydrogel. J Phys Chem. 1990;94:2689–96.
Hofer K, Mayer E, Johari GP. Glass liquid transition and calorimetric relaxation of glassy aqueous-solutions imbibed in poly(2-hydroxyethyl methacrylate)—a comparison with bulk behavior. J Phys Chem. 1991;95:7100.
Johari GP, Hallbrucker A, Mayer E. The glass liquid transition of hyperquenched water. Nature. 1987;330:552–3.
Johari GP, Hallbrucker A, Mayer E. Isotope effect on the glass-transition and crystallization of hyperquenched glassy water. J Chem Phys. 1990;92:6742–6.
Johari GP, Astl G, Mayer E. Enthalpy relaxation of glassy water. J Chem Phys. 1990;92:809–10.
Swenson J, Jansson H, Hedström J, Bergman R. Properties of hydration water, its role in protein dynamics. J Phys Condens Matter. 2007;19:205109.
Shinyashiki N, Yamamoto W, Yokoyama A, Yoshinari T, Yagihara S, Kita R, Ngai KL, Capaccioli S. Glass transitions in aqueous solution of protein (bovine serum albumin). J Phys Chem B. 2009;113:14448.
Kawai K, Suzuki T, Oguni M. Low-temperature glass transitions of quenched and annealed bovine serum albumin aqueous solutions. Biophys J. 2006;90:3732–8.
Doster W, Settles M. Protein-water displacement distributions. Biochim Biophys Acta. 2005;1749:173–86.
Doster W. The dynamical transition of proteins, concepts and misconceptions. Eur Biophys J. 2008;37:591–602.
Parak F, Knapp EW, Kucheida D. Protein dynamics Mössbauer spectroscopy on deoxymyoglobin crystals. J Mol Biol. 1982;161:177–94.
Doster W, Cusak S, Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989;337:754–6.
Chen S-H, Liu L, Fratini E, Baglioni P, Faraone A, Mamontov E. Observation of fragile-to-strong dynamic crossover in protein hydration water. Proc Natl Acad Sci USA. 2006;103:9012–6.
Khodadadi S, Pawlus S, Roh JH, Garcia Sakai V, Mamontov E, Sokolov AP. The origin of the dynamic transition in proteins. J Chem Phys. 2008;128:195106.
Khodadadi S, Pawlus S, Sokolov AP. Influence of hydration on protein dynamics: combining dielectric and neutron scattering spectroscopy data. J Phys Chem B. 2008;112:14273–80.
Tarek M, Tobias DJ. Role of protein-water hydrogen bond dynamics in the protein dynamical transition. Phys Rev Lett 2002;88:138101.
Ringe D, Petsko GA. The ‘glass transition’ in protein dynamics: what it is, why it occurs, and how to exploit it. Biophys Chem. 2003;105:667–80.
Miyazaki Y, Matsuo T, Suga H. Low-temperature heat capacity and glassy behavior of lysozyme crystal. J Phys Chem B. 2000;104:8044–52.
Teeter MM, Yamano A, Stec B, Mohanty U. On the nature of a glassy state of matter in a hydrated protein: relation to protein function. Proc Natl Acad Sci USA. 2001;98:11242–7.
Zanotti J-M, Gibrat G, Bellissent-Funel M-C. Hydration water rotational motion as a source of configurational entropy driving protein dynamics. Crossovers at 150 and 220 K. Phys Chem Chem Phys. 2008;10:4865.
Fenimore PW, Frauenfelder H, McMahon BH, Young RD. Bulk-solvent and hydration-shell fluctuations, similar to α- and β-fluctuations in glasses, control protein motions and functions. Proc Natl Acad Sci USA. 2004;101:14408–13.
Ngai KL. Dynamic and thermodynamic properties of glass-forming substances. J Non-Cryst Solids. 2000;275:7–51.
Casalini R, Ngai KL. Structural dependence of fast relaxation in glass-forming substances and correlation with the stretch exponent of the slow structural α-relaxation. J Non-Cryst Solids. 2001;293–295:318–26.
Angell CA. Ten questions on glassformers, and a real space ‘excitations’ model with some answers on fragility and phase transitions. J Phys Condens Matter. 2000;12:6463–75.
Sokolov AP, Kisliuk A, Novikov VN, Ngai KL. Observation of constant loss in fast relaxation spectra of polymers. Phys Rev B. 2001;63:172204.
Kisliuk A, Novikov VN, Sokolov AP. Constant loss in Brillouin spectra of polymers. J Polym Sci B. 2002;40:201–9.
Ngai KL. Why the fast relaxation in the picosecond to nanosecond time range can sense the glass transition. Philos Mag. 2004;84:1341–53.
Capaccioli S, Shahin Thayyil M, Ngai KL. Critical issues of current research on the dynamics leading to glass transition. J Phys Chem B. 2008;112:16035–49.
Ngai KL, Habasaki J, Leon C, Rivera A. Comparison of dynamics of ions in ionically conducting materials and dynamics of glass-forming substances: remarkable similarities. Z Phys Chem. 2005;219:47.
Nowaczyk A, Geil B, Hinze G, Böhmer R. Correlation of primary relaxations and high-frequency modes in supercooled liquids. II. Evidence from spin-lattice relaxation weighted stimulated-echo spectroscopy. Phys Rev E. 2006;74:041505.
Lusceac S, Vogel MR, Herbers CR. 2H and 13C NMR studies on the temperature-dependent water and protein dynamics in hydrated elastin, myoglobin and collagen. arXiv:0904.4424v1 [cond-mat.soft]. Accessed 28 April 2009.
Döß A, Paluch M, Sillescu H, Hinze G. From strong to fragile glass formers: secondary relaxation in polyalcohols. Phys Rev Lett. 2002;88:095701.
Morozov VN, Gevorkian SG. Low-temperature glass transition in proteins. Biopolymers. 1985;24:785.
Ngai KL, Capaccioli S, Shinyashiki The N. Protein “glass” transition, the role of the solvent. J Phys Chem B. 2008;112:3826.
Ngai KL, Capaccioli S, Shinyashiki N, Shahin Thayyil M. Recent progress in understanding relaxation in complex systems. J Non-Cryst Solids. in press.
Gainaru C, Fillmer A, Böhmer R. Surface activation by deeply supercooled hydration water in connective tissue proteins. J Phys Chem B 2009; Submitted.
Lorthioir C, Alegrıa A, Colmenero J. Out of equilibrium dynamics of poly(vinyl methyl ether) segments in miscible poly(styrene)-poly(vinyl methyl ether) blends. Phys Rev E. 2003;68:031805.
Cangiolosi D, Alegria A, Colmenero J. Dielectric relaxation of polychlorinated biphenyl/toluene mixtures: component dynamics. J Chem Phys. 2008;128:224508.
Urakawa O, Fuse Y, Hori H, Tran-Cong Q, Yano O. A dielectric study on the local dynamics of miscible polymer blends: poly(2-chlorostyrene)/poly(vinyl methyl ether). Polymer. 2001;42:765–73.
Miwa Y, Usami K, Yamamoto K, Sakaguchi M, Sakai M, Shimada S. Direct detection of effective glass transitions in miscible polymer blends by temperature-modulated differential scanning calorimetry. Macromolecules. 2005;38:2355–61.
Kahle S, Korus J, Hempel E, Unger R, Höring S, Schröter G, et al. Glass-transition cooperativity onset in a series of random copolymers poly(n-butyl methacrylate-stat-styrene). Macromolecules. 1997;30:7214–23.
Ngai KL. Correlation between β-relaxation and α-relaxation in the family of poly(n-butyl methacrylate-stat-styrene) random copolymers. Macromolecules. 1999;32:7140–6.
Miller JB, McGrath KJ, Roland CM, Trask CA, Garroway AN. Nuclear magnetic resonance study of polyisoprene/poly(vinylethylene) miscible blends. Macromolecules. 1990;23:4543–7.
Alegria A, Colmenero J, Ngai KL, Roland CM. Observation of the component dynamics in a miscible polymer blend by dielectric, mechanical spectroscopies. Macromolecules. 1994;27:4486.
Ngai KL, Roland CM. Component dynamics in polyisoprene/poly(vinylethylene) blends. Macromolecules. 1995;28:4033–5.
Chung G-C, Kornfield JA, Smith SD. Compositional dependence of segmental dynamics in a miscible polymer blend. Macromolecules. 1994;27:5729–41.
Sakaguchi T, Taniguchi N, Urakawa O, Adachi K. Calorimetric study of dynamical heterogeneity in blends of polyisoprene and poly(vinylethylene). Macromolecules. 2005;38:422–8.
Lodge TP, Wood ER, Haley JC. Two calorimetric glass transitions do not necessarily indicate immiscibility: the case of PEO/PMMA. J Polym Sci B. 2006;44:756–63.
Ngai KL, Roland CM. Models for the component dynamics in blends and mixtures. Rubber Chem Technol. 2004;77:579–90.
Roland CM, McGrath KJ, Casalini R. Dynamic heterogeneity in poly(vinyl methyl ether)/poly(2-chlorostyrene) blends. Macromolecules. 2006;39:3581–7.
Lutz TR, He Y, Ediger MD, Cao H, Lin G, Jones AA. Rapid poly(ethylene oxide) segmental dynamics in blends with poly(methyl methacrylate). Macromolecules. 2003;36:1724–30.
Ngai KL, Roland CM. Unusual component dynamics in poly(ethylene oxide)/poly(methyl methacrylate) blends as probed by deuterium NMR. Macromolecules. 2004;37:2817–22.
Ngai KL. Predicting the changes of relaxation dynamics with various modifications of the chemical and physical structures of glass-formers. J Non-Cryst Solids. 2007;353:4237–45.
Jin X, Zhang S, Runt J. Broadband dielectric investigation of amorphous poly(methyl methacrylate)/poly(ethylene oxide) Blends. Macromolecules. 2004;37:8110–5.
Doss A, Hinze G, Schiener B, Hemberger J, Böhmer R. Dielectric relaxation in the fragile viscous liquid state of toluene. J Chem Phys. 1997;107:1740.
Kudlik A, Benkhof S, Blochowicz T, Tschirwitz C, Rossler E. The dielectric response of simple organic glass formers. J Mol Liq. 1999;479:201.
Hensel-Bielowka S, Paluch M, Ngai KL. Emergence of the genuine Johari–Goldstein secondary relaxation in m-fluoroaniline after suppression of hydrogen-bond-induced clusters by elevating temperature and pressure. J Chem Phys. 2005;123:014502.
Adachi K, Fujihara I, Ishida Y. Diluent effects on molecular motions and glass transition in polymers. I. Polystyrene-toluene. J Polym Sci Polym Phys Ed. 1975;13:2155–71.
Rössler E, Sillescu H, Spiess HW. Deuteron NMR in relation to the glass transition in polymers. Polymer. 1985;26:203–7.
Floudas G, Steffen W, Fischer EW, Brown W. Solvents and polymer dynamics in concentrated polystyrene/toluene solutions. J Chem Phys. 1993;99:695.
Rizos AK, Johnsen RM, Brown W, Ngai KL. Component dynamics in polystyrene/bis(2-ethylhexyl) phthalate solutions by polarized and depolarized light scattering and dielectric spectroscopy. Macromolecules. 1996;28:5450–7.
Taniguchi N, Urakawa O, Adachi K. Calorimetric study of dynamical heterogeneity in toluene solutions of polystyrene. Macromolecules. 2004;37:7832–8.
Braun G, Kovacs AJ. Variations de la temperature de transition vitreuse du polystyrolene en fonction de la concentration en plastifiant. Compte Rendus. 1965;260:2217–20.
Plazek DJ, Riande E, Markovitz H, Raghupathi N. Concentration dependence of the viscoelastic properties of polystyrene-tricresyl phosphate solutions. J Polym Sci Polym Phys Ed. 1979;17:2189.
Savin DA, Larson AM, Lodge TP. Effect of composition on the width of the calorimetric glass transition in polymer-solvent and solvent-solvent mixtures. J Polym Sci B. 2004;42:1155–63.
Svanberg C, Bergman R, Jacobsson P. Secondary relaxation in confined and bulk propylene carbonate. Europhys Lett. 2003;64:358–63.
Acknowledgements
This study was supported at NRL by the Office of Naval Research, at the Università di Pisa by MIUR-FIRB 2003 D.D.2186 grant RBNE03R78E, and at Tokai University by Grant-in-Aid for Scientific Research (C)(19540429).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ngai, K.L., Capaccioli, S., Shahin Thayyil, M. et al. Resolution of problems in soft matter dynamics by combining calorimetry and other spectroscopies. J Therm Anal Calorim 99, 123–138 (2010). https://doi.org/10.1007/s10973-009-0500-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0500-y