Abstract
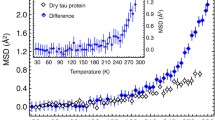
The dynamics of hydrated proteins and of protein crystals can be studied within a wide temperature range, since the water of hydration does not crystallize at low temperature. Instead it turns into an amorphous glassy state below 200 K. Extending the temperature range facilitates the spectral separation of different molecular processes. The conformational motions of proteins show an abrupt enhancement near 180 K, which has been called a “dynamical transition”. In this contribution various aspects of the transition are critically reviewed: the role of the instrumental resolution function in extracting displacements from neutron elastic scattering data and the question of the appropriate dynamic model, discrete transitions between states of different energy versus continuous diffusion inside a harmonic well, are discussed. A decomposition of the transition involving two motional components is performed: rotational transitions of methyl groups and small scale librations of side-chains, induced by water at the protein surface. Both processes create an enhancement of the observed amplitude. The onset occurs, when their time scale becomes compatible with the resolution of the spectrometer. The reorientational rate of hydration water follows a super-Arrhenius temperature dependence, a characteristic feature of a dynamical transition. It occurs only with hydrated proteins, while the torsional motion of methyl groups takes place also in the dehydrated or solvent-vitrified system. Finally, the role of fast hydrogen bond fluctuations contributing to the amplitude enhancement is discussed.







Similar content being viewed by others
References
Becker T, Smith J (2003) Energy resolution and dynamical heterogeneity effects on elastic incoherent neutron scattering from molecular systems. Phys Rev E 67:021904-1-8
Becker T, Hayward JA, Finney JL, Daniel RM, Smith J (2004) Neutron frequency windows and the protein dynamical transition. Biophys J 87:1436–1444
Bicout DJ, Zaccai G (2001) Protein flexibility from the dynamical transition, a force constant analyis. Biophys J 80:115-1123-8
Buchenau U, Zorn R (1992) A relation between fast and slow motions in glassy and liquid selenium. Europhys Lett 18:523–528
Cornicchi E, Onori G, Paciaroni A (2005) Picosecond time scale fluctuations of proteins in glassy matrices: the role of viscosity. Phys Rev Lett 95:158104-1-4
Daniel RM, Finney JL, Reat V, Dunn R, Ferrand M, Smith J (1999) Enzyme dynamics and activity, the time scale dependence of dynamical transitions. Biophys J 77:2184–219
Demmel F, Doster W, Petry W, Schulte A (1997) Vibrational frequencies as a probe of hydrogen bonds: thermal expansion and glass transition of myoglobin in mixed solvents. Eur Biophys J 26:327–335
Doster W (2005) Brownian oscillator analysis of molecular motions in biomolecules. In: Fitter J, Gutberlet T, Katsaras J (eds) Neutron scattering in biology. Springer series biological and medical physics, biomedical engineering, pp 461–482
Doster W (2006) Dynamic structural distributions in proteins. Physica B 385–386:831–834
Doster W, Settles M (1998) Dynamical transition of proteins, the role of hydrogen bonds. In: Bellissent-Funel M-C (ed) Hydration processes in biology (Les Houches lectures). IOS Press, Amsterdam, pp 177–190
Doster W, Settles M (2005) Protein–water displacement distributions. Biochim Biophys Act 1749:173–186
Doster W, Lüscher E, Bachleitner A, Dunau R, Hiebl M (1986) Thermal properties of water in myoglobin crystals and solutions at subzero temperatures. Biophys J 50:213–219
Doster W, Cusack S, Petry W (1989) Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature 337:754–756
Doster W, Cusack S, Petry W (1990) Dynamic instability of liquid-like motions in proteins. Phys Rev Lett 65:1083–1086
Doster W, Diehl M, Petry W, Ferrand M (2001) Elastic resolution spectroscopy: a method to study molecular motions in small biological samples. Physica B 301:65–68
Doster W, Diehl M, Gebhardt R, Lechner RE, Pieper J (2003) Time-of-flight elastic resolution spectroscopy: time domain analysis of weakly scattering samples. Chem Phys 292:487–494
Fenimore P W, Frauenfelder H, McMahon BH, Young RD (2005) Proteins are paradigms of stochastic complexity. Physica A 351:1–13
Ferrand M, Dianoux AJ, Petry W, Zaccai G (1993) Thermal motions and function of bacteriorhodopsin in purple membranes: effects of temperature and hydration studied by neutron scattering. Proc Natl Acad Sci USA 90:9668–9672
Fitter J, Lechner RE, Dencher NA (1997) Picosecond molecular motions in bacteriorhodopsin from neutron scattering. Biophys J 73:2126–2137
Gabel F (2005) Protein dynamics in solution and powder measured by incoherent elastic neutron scattering. Eur Biophys J 34:1–12
Gabel F, Bicout D, Lehnert U, Tehei M, Weik M, Zaccai G (2002) Protein dynamics, studied by neutron scattering. Q Rev Biophys 35:1–32
Götze W, Sjögren L (2005) Relaxation processes in supercooled liquids. Rep Prog Phys 55:241–376
Hayward JA, Finney JL, Daniel RM, Smith JC (2003) Molecular dynamics decomposition of temperature-dependent elastic neutron scattering by a protein solution. Biophys J 85:679–685
Keller H, Debrunner P (1980) Evidence for conformational and diffusional mean square displacements in frozen aqueous solution of oxy-myoglobin. Phys Rev Lett 45:68–71
Kleinert Th, Doster W, Leyser H, Petry W, Schwarz V, Settles M (1998) Solvent composition and viscosity effects on the kinetics of CO-binding to horse myoglobin. Biochem 37:717–733
Kurkal V, Daniel RM, Finney JL, Tehei M, Dunn RV, Smith J (2005) Enzyme activity and flexibility at very low hydration. Biophys J 89:1282–11287
Lichtengegger H, Doster W, Kleinert T, Birk A, Sepiol B, Vogl G (1999) Heme-solvent coupling, a Mössbauer study of myoglobin in sucrose. Biophys J 76:414–422
Magazu S, Maisano G, Migliardo F, Mondelli C (2004) Mean square displacement relationship in bioprotectant systems by elastic neutron scattering. Biophys J 86:3241–3249
Nakagawa H, Kmikubo I, Kanaya T, Kataoka M (2004) Protein dynamical heterogeneity derived from neutron incoherent elastic scattering. J Phys Soc Jpn 73:491–495
Paciaroni A, Cornicchi E, De Francesco A, Marconi M, Onori G (2006) Conditioning action of the environment on the protein dynamics studied through elastic neutron scattering. Eur Biophys J 35(7):591–599
Parak F, Achterhold K (2005) Protein dynamics on different timescales. J Phys Chem Solids 66:2257–2262
Parak F, Formanek H (1971) Untersuchung des Schwingungsanteils und des Kristallgitterfehleranteils des Temperaturfaktors in Myoglobin durch Vergleich von Mössbauerabsorptionsmessungen mit Röntgenstrukturdaten. Acta Crystallogr A 27:573–578
Parak F, Knapp EW (1984) A consistent picture of protein dynamics. PNAS USA 81:7088–7092
Parak F, Frovlov EN, Mössbauer RL, Goldanskij VI (1981) Dynamcis of metmyoglobin investigated by nuclear gamma-resonance absorption. J Mol Biol 145:237–249
Parak F, Fischer M, Graffweg E Formanek, H (1987) Distributions and fluctuations of protein structures investigated by X-ray analysis and Mössbauer spectroscopy. In: Clementi E, Chin S (eds) Structure and dynamics of nucleic acids, proteins and membranes. Plenum Press, New York, pp 139–148
Reat V, Zaccai G, Pfister C, Ferrand M (1997) Functional dynamics in purpel membrane. In: Cusack S, Büttner H, Ferrand M, Langan P, Timmins P (eds) Biological macromolecular dynamics. Adenine Press, New York, pp 117–122
Reat V, Dunn R, Ferrand M, Finney JL, Daniel RM, Smith JC (2000) Solvent dependence of dynamic transitions in protein solutions. Proc Natl Acad Sci USA 97:9961–9966
Roh JH, Novikov VN, Gregory RB, Curtis JE, Chowduri Z, Sokolov AP (2005) Onset of unharmonicity in protein dynamics. Phys Rev Lett 95:038101
Russo D, Perez J, Zanotti JM, Desmadril M, Durand D (2002) Dynamic transition associated with the thermal denaturation of a small beta protein. Biophys J 83:2792–2800
Sartor G, Mayer E, Johari GP (1994) Calorimetric studies of the kinetic unfreezing of molecular motions in hydrated lysozyme, hemoglobin and myoglobin. Biophys J 66:249–258
Serdyuk IN, Zaccai NR, Zaccai G (2007) Methods in molecular biophysics. Cambridge University Press, London, p 957
Settles M, Doster W (1997) Iterative calculation of the vibrational density of states from incoherent neutron scattering data with the account of double scattering. In: Buettner H, Cusack S, Ferrand M, Lagan P, Timmins P (eds) Biological macromolecular dynamics. Adenine Press, New York, pp 3–9. ISBN 0-940030-49-7
Sing GP, Parak F, Hunklinger S, Dransfeld K (1981) Role of adsorbed water in the dynamics of metmyoglobin. Phys Rev Lett 47:685–688
Smith J (1991) Protein Dynamics, a comparison of simulations with inelastic neutron scattering experiments. Q Rev Biophys 24:227–291
Smith DJ, Ferrand M, Hery S, Dunn RJ, Finney JL (1998) Enzyme activity below the dynamical transition. Biophys J 75:2504–2507
Tarek M, Tobias D (2002) Single particle and collective dynamics of protein hydration water. Phys Rev Lett 89:13801–13804
Tarek M, Tobias T (2002) The role of protein–water hydrogen bonds in the dynamical transition of proteins. Phys Rev Lett 88:381011–381013
Tehei M, Madern D, Franzetti B, Zaccai G (2005) Neutron scattering reveals the dynamic basis of protein adaption to extreme temperature. J Bio Chem 280:40974–40979
Tehei M, Smith JC, Monk C, Ollivier J, Oettl M, Kurkal V, Finney JL, Daniel RM (2006a) Dynamics of immobilized and native E. coli dihhydrofolate reductase by quasi-elastic neutron scattering. Biophys J 90:1090–1097
Tehei M, Daniel R, Zaccai G (2006b) Fundamental and biotechnological application of neutron scattering measurements for macromolecular dynamics. Eur Biophys J 35:551–558
Tournier AL, Smith JC (2003) Principal components of the protein dynamical transition. Phys Rev Lett 91(20):208106-1–208106-4
Tournier AL, Xu J, Smith JC (2003) Solvent caging of internal motions in myoglobin at low temperatures. Phys Chem Commun 6(2):6–8
Tsai AM, Neumann DA, Bell LN (2000) Molecular dynamics of solid-state lysozyme as affected by glycerol and water, a neutron scattering study. Biophys J 79:2728–2732
Zaccai J (2000) How soft is a protein? A protein force constant measured by neutron scattering. Science 288:1604–1607
Acknowledgments
This work was supported by a grant of the Deutsche Forschungsgemeinschaft within the SFB 533, light-induced processes in biopolymers. Excellent technical support by the instrument responsibles including the Institut Laue Langevin and numerous discussions with Prof. Wolfgang Götze are gratefully acknowleded. The NMR experiments were performed by Prof. Franz Fujara.
Author information
Authors and Affiliations
Corresponding author
Additional information
Advanced neutron scattering and complementary techniques to study biological systems. Contributions from the meetings, “Neutrons in Biology”, STFC Rutherford Appleton Laboratory, Didcot, UK, 11–13 July and “Proteins At Work 2007”, Perugia, Italy, 28–30 May 2007.
Rights and permissions
About this article
Cite this article
Doster, W. The dynamical transition of proteins, concepts and misconceptions. Eur Biophys J 37, 591–602 (2008). https://doi.org/10.1007/s00249-008-0274-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-008-0274-3