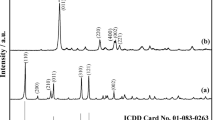
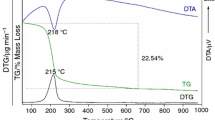
Abstract
The non-isothermal kinetics of dehydration of AlPO4·2H2O was studied in dynamic air atmosphere by TG–DTG–DTA at different heating rates. The result implies an important theoretical support for preparing AlPO4. The AlPO4·2H2O decomposes in two step reactions occurring in the range of 80–150 °C. The activation energy of the second dehydration reaction of AlPO4·2H2O as calculated by Kissinger method was found to be 69.68 kJ mol−1, while the Avrami exponent value was 1.49. The results confirmed the elimination of water of crystallization, which related with the crystal growth mechanism. The thermodynamic functions (ΔH*, ΔG* and ΔS*) of the dehydration reaction are calculated by the activated complex theory. These values in the dehydration step showed that it is directly related to the introduction of heat and is non-spontaneous process.




Similar content being viewed by others
References
Lagno F, Demopoulos GP. Synthesis of hydrated aluminum phosphate, AlPO4·1.5H2O (AlPO4−H3), by controlled reactive crystallization in sulfate media. Ind Eng Chem Res. 2005;44:8033–8.
Siva Kumar V, Padmasri AH, Satyanarayana CVV, Ajit Kumar Reddy I, David Raju B, Rama Rao KS. Nature and mode of addition of phosphate precursor in the synthesis of aluminum phosphate and its influence on methanol dehydration to dimethyl ether. Catal Commun. 2006;7:745–51.
Guti′errez-Mora F, Goretta KC, Singh D, Routbort JL, Sambasivan S, Steiner KA, et al. High-temperature deformation of amorphous AlPO4-based nano-composites. J Eur Ceram Soc. 2006;26:1179–83.
Mostafa MR, Ahmed FSh. Characterization and catalytic behaviour of Co3(PO4)2–AlPO4 catalysts. Adsorb Sci Technol. 1998;16:285–93.
Ahmed FSh, Mostafa MR, Kiwan HH. Characterization and catalytic activity of Cr2O3–Al2O3/AlPO4 catalysts. Adsorb Sci Technol. 2000;18:709–17.
Mohamed FSh, Kiwan HH, Mostafa MR. Effect of chemical composition on the structure and catalytic behaviour of AlPO4 and Al2O3–AlPO4 mixed catalysts. Adsorb Sci Technol. 2002;20:131–40.
Arjona AM, Franco MAA. Kinetics of the thermal dehydration of variscite and specific surface area of the solid decomposition products. J Therm Anal Calorim. 1973;5:319–28.
Stojakovic D, Rajic N, Sajic S, Logar NZ, Kaucic V. A kinetic study of the thermal degradation of 3-methylaminopropylamine inside AlPO4-21. J Therm Anal Calorim. 2007;87:337–43.
Boonchom B, Youngme S, Srithanratana T, Danvirutai C. Synthesis of AlPO4 and kinetics of thermal decomposition of AlPO4·H2O–H4 precursor. J Therm Anal Calorim. 2007;91:511–6.
Kissinger HE. Reaction kinetics in differential thermal analysis. J Anal Chem. 1957;29:1702–6.
Vlaev LT, Nikolova MM, Gospodinov GG. Non-isothermal kinetics of dehydration of some selenite hexahydrates. J Solid State Chem. 2004;177:2663–9.
Vlase T, Vlase G, Brita N, Doca N. Comparative results of kinetic data obtained with different methods for complex decomposition steps. J Therm Anal Calorim. 2007;88:631–5.
Colthup NB, Daly LH, Wiberley SE. Introduction to Infrared and Raman spectroscopy. New York: Academic Press; 1964.
Frost RL, Weier ML, Martens WN, Henry DA, Mills SJ. Raman spectroscopy of newberyite, hannayite and struvite. Spectrochim Acta. 2005;62A:181–8. (and Ref. therein).
Scaccia S, Carewska M, Di Bartolomeo A, Prosini PP. Thermoanalytical investigation of nanocrystalline iron (II) phosphate obtained by spontaneous precipitation from aqueous solutions. Thermochim Acta. 2002;383:141–5. (and Ref. therein).
Vlaev LT, Georgieva VG, Genieva SD. Products and kinetics of non-isothermal decomposition of vanadium(IV) oxide compounds. J Therm Anal Calorim. 2007;88:805–12.
Zhang K, Hong J, Cao G, Zhan D, Tao Y, Cong C. The kinetics of thermal dehydration of copper(II) acetate monohydrate in air. Thermochim Acta. 2005;437:145–9.
Budrugeac P. The Kissinger law and the IKP method for evaluating the non-isothermal kinetic parameters. J Therm Anal Calorim. 2007;89:143–51.
Gabal MA. Kinetics of the thermal decomposition of CuC2O4−ZnC2O4 mixture in air. Thermochim Acta. 2003;402:199–208.
Cordes HM. Preexponential factors for solid-state thermal decomposition. J Phys Chem. 1968;72:2185–9.
Criado JM, Pérez-Maqueda LA, Sánchez-Jiménez PE. Dependence of the preexponential factor on temperature. J Therm Anal Calorim. 2005;82:671–5.
Anilkumar GM, Sung Y-M. Phase formation kinetics of nanoparticle-seeded strontium bismuth tantalate powder. J Mater Sci. 2003;38:1391–6.
Zhao MS, Song XP. Synthesizing kinetics and characteristics for spinel LiMn2O4 with the precursor using as lithium-ion battery cathode material. J Power Source. 2007;164:822–8.
Zhang Y, Lv M, Chen D, Wu J. Leucite crystallization kinetics with kalsilite as a transition phase. Mater Lett. 2007;61:2978–81.
Singh BK, Sharma RK, Garg BS. Kinetics and molecular modeling of biologically active glutathione complexes with lead(II) ions. J Therm Anal Calorim. 2006;84:593–600.
Ŝesták J. Thermodynamical properties of solids. Prague: Academia; 1984.
Young D. Decomposition of solids. Oxford: Pergamon Press; 1966.
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M, Anal J. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. Appl Pyrol. 2008;81:253–62.
Acknowledgements
The authors would like to thank the Chemistry Department, Khon Kaen University for providing research facilities. This work is financially supported by King Mongkut’s Institute of Technology Ladkrabang (KMITL) and the Center excellence for Innovation in Chemistry: Postgraduate Education and Research Program in Chemistry (PERCH-CIC), Ministry of Education, Thailand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boonchom, B., Danvirutai, C. Kinetics and thermodynamics of thermal decomposition of synthetic AlPO4·2H2O. J Therm Anal Calorim 98, 771–777 (2009). https://doi.org/10.1007/s10973-009-0292-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0292-0