Abstract
The isothermal heat of hydration of MgSO4 hydrates was studied by humidity controlled calorimetry. Two hydrates, starkeyite (MgSO4·4H2O) and a mixture of MgSO4 hydrates with summary 1.3 mol H2O were investigated. The solid-gas reactions were initiated at 30°C and 85% relative humidity. The heat of hydration was determined in a circulation cell in the calorimeter C80 (Setaram). The crystal phases formed after the hydration process were analyzed by thermogravimetry (TG) and X-ray powder diffraction (XRD).
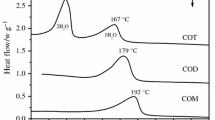
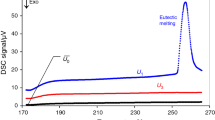
Starkeyite reacted with the water vapour to the thermodynamic stable epsomite and the MgSO4 hydrate mixture with 1.3 mol water to hexahydrite. The hydration heats of starkeyite and the mixture were determined to be −169±3 and −257±5 kJmol−1, respectively.
Similar content being viewed by others
References
E. A. Levitskij, Y. I. Aristov, M. M. Tokarev and V. N. Parmon, Sol. Energy Mat. Sol. Cells, 44 (1996) 219.
J. Jänchen, D. Ackermann, H. Stach and W. Brösicke, Sol. Energy, 76 (2004) 339.
M. Steiger and W. Dannecker, R. Snethlage, Ed., Jahresberichte Steinzerfall: Steinkonservierung, Ernst & Sohn, Berlin 1993, p. 123.
J. Pühringer, Int. Zeitschrift für Bauinstandsetzen und Baudenkmalpflege, 5 (2002) 515.
H. Stach, D. Ackermann, W. Brösicke, J. Jänchen and E. Weiler, Schlussbericht, Entwicklung und Charakterisierung von mikroporösen Festkörpern für die adsorptive Langzeitspeicherung von Niedertemperaturwärme, Förderkennzeichen 0329525C, BMWi, 2001, p. 79.
H. Stach, J. Mugele, J. Jänchen and E. Weiler, Adsorption, 11 (2005) 393.
L. G. Gordeeva, I. S. Glaznev and Y. I. Aristov, Russ. J. Phys. Chem., 77 (2003) 1715.
Gmelins Handbuch der Anorganischen Chemie, Syst. No. 27, Magnesium, Weinheim/Bergstraäe, 1952, p. 217.
S. J. Chipera, D. T. Vaniman, D. L. Bish, J. W. Carey and W. C. Feldman, Lunar Planet. Sci. XXXVI (2005) Abstract #1497.
D. T. Vaniman, D. L. Bish, S. J. Chipera, C. I. Fialips, J. W. Carey and W. C. Feldman, Nature, 431 (2004) 663.
A. Juling, D. Kirchner, S. Brügerhoff, K. Linnow, M. Steiger, A. E. Jarad and G. Gülker, Proceedings of the 10th International Congress on Deterioration and Conservation of Stone, D. Kwiatkowski and R. Löfvendahl, Eds, ICOMOS, Stockholm 2004, Vol. 1, p. 190.
C. W. DeKock, United States Bureau of Mines, Information Circular 9081, 1986, pp. 39–44.
W. H. Bauer, Acta Cryst., 15 (1962) 815.
J. Paulik, F. Paulik and M. Arnold, Thermochim. Acta, 50 (1981) 105.
H.-H. Emons, G. Ziegenbalg, R. Naumann and F. Paulik, J. Therm. Anal. Cal., 36 (1990) 1265.
S. J. Chipera and D. T. Vaniman, Geochim. Cosmochim. Acta, 71 (2007) 246.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Posern, K., Kaps, C. Humidity controlled calorimetric investigation of the hydration of MgSO4 hydrates. J Therm Anal Calorim 92, 905–909 (2008). https://doi.org/10.1007/s10973-007-8640-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-007-8640-4