Abstract
The goal of this research is to create a perovskite ceramic with electrical and dielectric properties appropriate for energy storage, medical uses, and electronic devices. A bismuth ferric titanate, Bi0.7Ba0.3(FeTi)0.5O3, doped with barium and crystalline, was effectively synthesized at the A-site via sol–gel synthesis. A rhombohedral structure emerged in the R3́C space group, which was confirmed by room-temperature X-ray studies. An average grain size of 263 nm and a homogeneous grain distribution and chemical composition were confirmed by the results of scanning electron microscopy (SEM) and energy dispersive X-ray analysis (EDX). The study established a clear relationship between temperature, frequency, and the electrical properties of the material. Impedance spectroscopy and electrical modulus measurements, performed in the frequency range of 1 kHz to 1 MHz and at temperatures ranging from 200 K to 360 K, demonstrated a non-Debye type of relaxation. Furthermore, once the material was produced at various temperatures, its frequency-dependent electrical conductivity was examined using Jonscher’s law. The results demonstrate that barium doping significantly improves the electrical conductivity and dielectric properties compared to pure BiFeTiO₃. Over the complete temperature range, consistent conduction and relaxation mechanisms were discovered. These findings suggest that the chemical may find widespread applicability across a broad temperature range, including electrical fields and capacitors.
Graphical Abstract

Highlights
-
Successful Synthesis: A perovskite ceramic, Bi0.7Ba0.3(FeTi)0.5O3, was synthesized using the sol–gel method, doped with barium at the A-site.
-
Structural Properties: X-ray diffraction confirmed a rhombohedral structure in the R3́C space group, with an average grain size of 263 nm, as analyzed through SEM and EDX techniques.
-
Enhanced Electrical and Dielectric Properties: Barium doping significantly improved the electrical conductivity and dielectric properties of the material compared to undoped BiFeTiO₃, making it suitable for energy storage and electronic applications.
-
Non-Debye Relaxation Behavior: Impedance spectroscopy and electrical modulus analysis in the frequency range of 1 kHz to 1 MHz and temperature range of 200–360 K revealed non-Debye type relaxation.
-
Wide Applicability: The study identified stable conduction and relaxation mechanisms over a broad temperature range, suggesting potential use in capacitors and electrical fields across various temperature conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Because of its unique crystal structure, perovskite ceramics have become an interesting class of materials with a wide range of industrial applications. These ceramics, which get their name from the naturally occurring mineral perovskite, generally have the general formula ABX3, where A can be an alkali metal (Ca, Sr, Ag, Ba) or a lanthanide (La, Bi), and B can be either a magnetic transition metal element (Co, Ni, Fe, or Cr) or a non-magnetic alkaline-earth element (Nb, Mo, W, and Ti), and X can be an anion [1,2,3,4,5,6]. The versatility in their composition and structure allows for a broad spectrum of properties, making perovskite ceramics highly sought after for numerous industrial applications. In recent years, perovskite ceramics have gained significant attention in the field of electronics and energy [7,8,9,10,11,12,13,14] due to their unique combination of properties, such as high dielectric constant, tunable band-gap, and ferroelectric behavior. These characteristics make them ideal candidates for a wide range of applications, including energy storage, sensors, and particularly in the fabrication of solar cells. The efficiency of perovskite photovoltaics, coupled with their low production costs, has driven substantial interest in their development and commercialization, positioning them as a promising alternative to traditional silicon-based solar cells. The unique electronic and optical properties of perovskite ceramics make them promising candidates for next-generation solar energy conversion technologies. Beyond renewable energy, perovskite ceramics find utility in catalysis, sensing, and electronic devices. Their tunable properties, excellent stability, and catalytic activity make them valuable in industrial processes. Perovskite-based sensors have shown sensitivity to various gases and ions, contributing to advancements in environmental monitoring and safety applications [15, 16]. The perovskite compound, bismuth barium ferric titanate, denoted as BiFeO3, has been successfully synthesized using a conventional ceramic technology approach, as documented in the literature. Through a range of experimental techniques, the material underwent comprehensive characterization. X-ray diffraction analysis indicated a rhombohedral crystal structure. Additionally, a dielectric study demonstrated that the ceramic exhibits a low tangent loss and a high dielectric constant at room temperature. Barium doping in perovskite ceramics, specifically in bismuth ferric titanate (BiFeTiO₃), plays a crucial role in enhancing the material’s properties. The substitution of barium, which has a similar ionic radius to bismuth, can significantly improve the material’s electrical and dielectric performance. This doping alters the crystal structure and charge distribution, leading to enhanced electrical conductivity and dielectric constants, which are critical for high-performance capacitors and energy storage devices [17]. Studies have shown that barium doping not only improves the material’s electrical properties but also stabilizes its ferroelectric behavior [18]. Theoretical models support that barium doping enhances the polarization and dielectric response by reducing lattice distortions and improving ionic conductivity [19].
Upon closer examination, it was observed that the material displays space charge polarization and follows the Maxwell–Wagner dielectric mechanism at low frequencies and elevated temperatures. The confirmation of ferroelectricity was achieved through the analysis of polarization-electric field (PE) loops. Impedance plots also showed the behavior of semiconductors at high temperatures. Notably, a non-Debye kind of dielectric relaxation was shown by Nyquist plots analysis, 10 underscoring the contribution of both grains and grain borders to the resistive and capacitive properties of the material [20]. In the pursuit of advancing materials with precisely tuned electrical properties, this study unveils a detailed exploration into the dielectric dynamics of Bi0.7Ba0.3(FeTi)0.5O3 ceramic. The complex structure, which is the result of deliberate substitutions of Ba, Fe, and Ti into the perovskite lattice, adds unique characteristics to the electrical behavior of the material. Our research focuses on using impedance spectroscopy, a potent analytical tool, to examine the mechanisms controlling the ceramic’s electrical conduction and to reveal the intricate structural details of the material.
This work aims to not only understand the underlying interactions that control the material’s dielectric characteristics but also to provide important new information for future developments in electronic and technical applications. By means of exhaustive analysis, our objective is to enhance our comprehension of the complex relationship between structure and conduction mechanisms in prepared perovskite, thereby opening up new avenues for utilization in the field of advanced materials.
2 Materials and synthesis technique
The Bi0.7Ba0.3(FeTi)0.5O3 perovskite ceramic was synthesized using a sol–gel method [21, 22]. Initially, high-purity bismuth nitrate (Bi(NO₃)₃·5H₂O), barium carbonate (BaCO₃), iron oxide (Fe₂O₃), and titanium oxide (TiO₂) were mixed in the required molar ratios in distilled water at 70 °C with continuous stirring. Citric acid was added to aid in the dissolution and complexation of metal cations, followed by ethylene glycol to facilitate polymerization. After ~6 h, the resulting gel was dried at 350 °C for 8 h. To decompose organic residues and remove impurities, the dried gel was initially calcined at 500 °C for 4 h. Following this, the material underwent second calcination at 800 °C, 900 °C, and 1000 °C for 4 h at each temperature to further promote phase formation. After each calcination step, the material was ground, dried, pressed into pellets, and then subjected to a final sintering process at 1100 °C for 16 h. This sintering step was critical for achieving a pure and homogeneous perovskite ceramic structure. This multistep thermal treatment ensures the effective incorporation of oxides and the formation of a well-defined perovskite phase.
The X-ray diffraction (XRD) pattern was obtained using a two-circle automatic diffractometer, the “Panalytical X’Pert Pro System,” operating at a copper wavelength (λ = 1.5406 Å). A nickel filter was used to get rid of the Kβ radiation. In Bragg-Brentano geometry, the measurement was carried out between an angle range of 10 ≤ 2θ ≤ 70° using a diverging beam and a 0.016° step with a 16 s counting time per step. Using a Philips XL 30 microscope equipped with an electron gun and operating at a 15 kV accelerating voltage, the pelletized sample was analyzed morphologically by scanning electron microscopy (SEM). A disk-shaped sample with dimensions of 10.51 mm in diameter and 1.48 mm in thickness was used to characterize the electrical and dielectric properties. Using an Agilent 4294 A precision impedance analyzer in Cp-Rp (capacitance in parallel with resistance) mode, measurements were made at different temperatures in the 100 Hz to 1 MHz frequency range.
3 Analysis and experimental results
3.1 Structural and morphological analysis
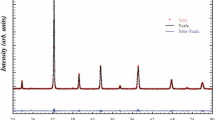
Figure 1 shows the room temperature X-ray diffraction (XRD) pattern of the calcined powder of Bi0.7Ba0.3(FeTi)0.5O3. The compound demonstrates a distinct crystallization pattern, with a perovskite phase being recognized as the main phase. The diffraction peaks of the compound were effectively indexed in the R3́C rhombohedral symmetry, consistent with the standard pattern provided by PDF Card No. [86-1518]. The sample exhibits high, sharp diffraction peaks that indicate a pure crystalline phase, indicating excellent crystallization. For structural refinement, the FULLPROF program and the Rietveld method were employed. The enhanced cell characteristics, unit cell volume, and reliability factors (goodness of fit χ2, profile factor Rp, weighted profile factor Rwp, and weighted profile factor RF) acquired through the use of Rietveld refinement are presented in Table 1. These numbers highlight the excellent quality, fit accuracy, and refinement accuracy attained. At 1100 °C, our XRD analysis confirmed the formation of a pure rhombohedral phase, characteristic of the Bi0.7Ba0.3(FeTi)0.5O3 composition. This single-phase formation and the rhombohedral crystal system indicate that the substitution of Ba and Ti into the BiFeO₃ lattice effectively stabilizes this structure [23, 24]. In contrast, pure BiFeTiO₃, as documented in the literature, typically crystallizes in an orthorhombic structure. The difference in crystal structure between pure BiFeTiO₃ and our doped composition (Bi0.7Ba0.3(FeTi)0.5O3) highlights the impact of Ba and Ti substitution. The incorporation of these elements induces a structural transition from the orthorhombic phase of BiFeTiO₃ to the rhombohedral phase observed in our doped ceramic.
Table 2 displays the X-ray diffraction statistics of the Bi0.7Ba0.3(FeTi)0.5O3 sample, emphasizing the interplanar spacing (d), Bragg locations (2θ and θ), and Miller indices. The crystalline structure of the sample was verified by the observation of diffraction peaks within the 2θ range of 20–70°. These data are essential for ascertaining the exact crystallographic characteristics and verifying the rhombohedral phase’s development. The following formulas [25] can be used to calculate the bulk density (Db), X-ray density (Dx), and porosity (P) of the material in order to further characterize it:
where m is the sample’s mass, r is the radius of the sample’s circular surface area, and ℎ is its thickness.
where M is the molar mass of the sample, N is the Avogadro number, and an is the lattice cell.
Based on the XRD peaks, the average particle size was calculated using the Scherer formula [26].
where δ is the Bragg angle, β is the corrected full-width half maximum of the XRD peaks, and λ is the X-ray wavelength. Grain size distribution was determined to be roughly DXRD = 23.5 nm. In addition, β is defined as β2 = β2m − β2s. βm is the experimental full width at half maximum (FWHM) and βs is the FWHM of a standard silicon sample. We also utilize the Williamson-Hall approach, which is represented by the following formula [27] to calculate the average crystallite size:
Where β the entire width at half maximum of the XRD peaks and denotes the strain. The DWH crystallite size is determined by calculating the intercept of the linear fit shown in Fig. 2. The data for crystallite size are summarized in Table 3. The results show that the particle size generated by the Williamson Hall technique is larger than the DXRD crystallite size predicted without accounting for the deformation effect (ε = 0).
This discrepancy can be explained by the widening caused by the deformation, which has a value of 0.14 percent. In addition, Table 4 displays the temperature parameters (ADP), occupation factors, and atomic locations for our sample that crystallized into a rhombohedral phase. The fractional coordinates provide the exact locations of the oxygen, Fe/Ti, and Bi/Ba atoms in the crystal lattice, and the occupation factors show how much of each site is occupied, which is important information for figuring out the crystallographic arrangement and material characteristics. As shown in Fig. 3a, we used scanning electron microscopy to investigate the general morphological properties of our sample. The SEM images demonstrate the homogeneous and thick grain morphology. The average grain size, which was determined using the average distribution of particles, is summarized in Table 3. It’s interesting to note that the crystallite size as evaluated by Scherrer DXRD techniques is substantially smaller than the grain size as reported by SEM. The discovery that each grain seen in a SEM is composed of many crystallites explains this discrepancy. Figure 3b presents a semi-quantitative analysis using energy dispersive spectroscopy (EDS). The item’s chemical composition can be more easily ascertained using this method. It is confirmed that the compositions of the generated samples, free of contaminants, match the fundamental stoichiometric compositions. It is acknowledged that only O, Bi, Ba, Fe, and Ti can produce any given peak. The elemental makeup of the produced sample and the precise peak energies found using energy-dispersive X-ray spectroscopy are summarized in Table 5. For the purpose of identifying and measuring the elements in the sample, the characteristic peak energies for Bi, Ba, Fe, Ti, and O are listed in kilo-electron volts (keV) in the table.
3.2 Analysis using impedance spectroscopy
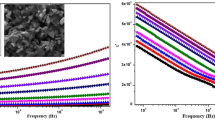
Changes in resistance, admittance, capacitance, and other system parameters in response to low-amplitude, variable-frequency stimulation are measured by impedance spectroscopy [28]. The real (Z′) component of impedance of the resulting perovskite fluctuates throughout a wide frequency and temperature range, as seen in Fig. 4. The compound Bi0.7Ba0.3(FeTi)0.5O3 exhibits both ferroelectric and semiconductor properties, as evidenced by its impedance behavior and dielectric characteristics. It was discovered that Z′ remains constant up to a certain lower frequency, after which it decreases with increasing frequency, leading to enhanced material conductivity. This can be attributed to the fact that at lower frequencies, impedance is dominated by the material’s bulk properties, such as space charge polarization and resistive elements. As frequency increases, space charges migrate more freely, reducing impedance and increasing conductivity. The decreasing trend of Z′ with temperature is indicative of the material’s semiconducting nature, specifically its negative temperature coefficient of resistance (NTCR), where conductivity increases with temperature due to thermally activated charge carriers. Additionally, the material’s ability to sustain spontaneous polarization and respond to an external electric field highlights its ferroelectric characteristics. Therefore, the compound can be classified as both a ferroelectric and a semiconductor, with its properties varying depending on the application and specific conditions.
Moreover, Z′ converges at high-frequency regions and fluctuates in a temperature-dependent manner. The decreasing trend of Z′ with temperature indicates the semiconducting nature of the materials, often known as the negative temperature coefficient of resistance or NTCR. Higher frequencies allow Z′ values to combine due to potential space charge release [29, 30] and a decrease in the material’s barrier properties, which is accounted for by space charge polarization. This trend aligns with results for various materials reported in the literature [17, 20]. In Fig. 5a, the Z versus frequency graph is displayed. Peaks appear at lower temperatures and get flatter at higher ones as temperatures rise, indicating that peaks are getting wider. This behavior can be attributed to the increasing mobility of charge carriers at higher temperatures. As temperature increases, the thermal energy allows more charge carriers, such as ions and electrons, to participate in conduction processes. This increased mobility results in a broader distribution of relaxation times, causing the peaks to flatten and widen. Additionally, the shift of Zmax into higher frequency domains as temperature rises indicates a larger loss tangent, suggesting enhanced energy dissipation in the material. This spectrum confirms the presence of temperature-dependent electrical relaxation processes, where at low temperatures, ions, stationary species, and electrons dominate, while at higher temperatures, defects and vacancies contribute significantly to the material’s electrical behavior [31, 32]. The values for the relaxation time and frequency are listed in Table 6. The observed decrease in relaxation length (τ) with rising temperature, alongside the increase in relaxation frequency (Fmax), can be explained by the enhanced mobility of charge carriers as the temperature increases. As temperature rises, the thermal energy available to the charge carriers increases, allowing them to respond more quickly to the applied electric field, which in turn reduces the relaxation time (τ). Concurrently, the increase in Fmax indicates that the relaxation processes occur at higher frequencies, consistent with a faster polarization response. This behavior suggests that the polarization mechanism in the compound is heavily influenced by space charge effects, where the relaxation is dominated by the migration and realignment of these charges under an electric field.
The link between relaxation time and frequency is expressed as follows:
Figure 5b displays the normalized imaginary components (Z″/Zmax) of the impedance with respect to frequency at various temperatures. One obvious trend that points to the presence of a temperature-dependent relaxation mechanism is the frequent shifting of peaks. The little frequency motion that was observed at different temperatures lends credence to this notion. The Arrhenius relation can be used to simulate the temperature dependence of the relaxation frequency (Fmax) in the following manner [33, 34]:
The pre-exponential term, activation energy, and Boltzmann constant are denoted by the symbols f0, Ea, and KB, respectively. The variation of Ln (Fmax) as a function of 1000/T is shown in Fig. 5c. The linear fit of the curve predicts an activation energy (Ea) value of 0.41 eV. The relationship between Z′ and Z″ at different temperatures (200–360 K) over the frequency range of 1 kHz–1 MHz is shown by the Nyquist plot, as shown in Fig. 6. This graphic clearly displays semicircle arcs in the impedance spectra at all temperatures. These semicircles demonstrate conduction along grain boundaries, suggesting that the bulk of the sample’s conduction process is accounted for by grain boundary contribution. As temperature rises, the radius of the semicircular arc decreases and its center point moves closer to the center of the axis. This pattern implies that the structure being studied has a non-Debye type relaxation and a relaxation time distribution [35]. This result is validated by fitting theoretical data with empirically collected impedance data using the Z-View software circuit model [36]. The chosen equivalent circuit configuration is of the kind (Rg + Rgb//CPEgb), as shown in Fig. 7. In this case, the grain border resistances are denoted by Rg and Rgb, respectively, while the grain boundary constant phase element is denoted by CPEgb. The impedance response of a constant phase element (CPE) is defined as follows [37, 38]:
where the frequency-independent CPE parameters were Q and α.The computed values of Rg, Rgb, CPEgb, and α are shown in Table 7 for each temperature. As previously mentioned, the expected Rg values significantly outweigh the Rgb values, indicating that the grain boundary contribution is primarily responsible for the conduction process in the sample. The table shows that the grain resistance Rg decreases with increasing temperature. By including charge carrier mobility in the conduction process, this behavior can be explained [39]. It also indicates that the substance is a semiconductor. In addition, according to the Table 6, the resistance of the grain (Rg) decreases steadily from 1110.25 kΩ at 200 K to 51.35 kΩ at 360 K, indicating a trend towards increased conductivity with higher temperatures. Conversely, the grain boundary resistance (Rgb) exhibits a less pronounced decrease from 1580.28 kΩ at 200 K to 54.83 kΩ at 360 K, suggesting a more stable interface response to temperature variations. The constant phase element for the grain boundary (CPEgb) decreases gradually from 12.52 × 10−11 F at 200 K to 8.16 × 10−11 F at 360 K, indicating changes in the electrical properties of the grain boundary structure. The α parameter, associated with the non-ideal capacitive behavior of CPE elements, shows a slight decrease from 0.82 at 200 K to 0.60 at 360 K, suggesting a shift towards more ideal capacitive behavior at higher temperatures. These findings highlight the complex temperature-dependent electrical characteristics of the sample, crucial for understanding its behavior in various applications requiring precise impedance control.
3.3 Analysis of AC conductivity
Figure 8 illustrates the frequency dependence of AC conductivity for our ceramic material at various temperatures. The plot clearly demonstrates low-frequency dispersion, where the conductivity increases with frequency, and this behavior becomes more pronounced at higher temperatures. As shown in the graph, conductivity indeed increases with both temperature and frequency. This trend can be attributed to several factors. At low frequencies, the conductivity is dominated by DC conduction mechanisms, including space charge polarization and the movement of trapped charges. As the frequency increases, these mechanisms become less dominant, and the AC conductivity, which is sensitive to the mobility of charge carriers and the material’s dielectric response, becomes more significant. The convergence of the curves at higher frequencies indicates that the material’s response to the applied electric field becomes more uniform, and the conductivity levels off. The increase in conductivity with temperature is related to the enhanced thermal activation of charge carriers. Higher temperatures provide more thermal energy to overcome energy barriers, thus facilitating increased charge mobility and consequently higher conductivity. The observed trend is consistent with Jonscher’s power law, which describes the frequency-dependent behavior of conductivity and can be used to analyze the material’s conduction mechanisms more thoroughly. This law suggests that the AC conductivity increases with frequency due to the reduction in the material’s impedance and the increased mobility of charge carriers [40, 41].
The detailed analysis of these results helps to understand the underlying conduction processes in the material, particularly how temperature and frequency affect charge mobility and conductivity. Additional results and discussions are included in the revised manuscript to provide a more comprehensive understanding of these behaviors. The conductivity can be examined using Jonscher’s power law [42, 43]:
Where n is the exponent factor, 0 < s < 1, and σdc is the direct current conductivity at low frequencies and certain temperatures. A is a constant that is dependent on temperature. It demonstrates that the typical charge accumulation behavior of ωs is diminished and that the conductivity increases with frequency. A frequency-independent flat response is seen at lower frequencies and higher temperatures. The difference in conductivity at lower frequencies can be recognized thanks to the polarization effect at the electrode and dielectric interface. The increase in AC conductivity with frequency, despite the decrease in accumulated charges, can be attributed to the fact that at higher frequencies, the material’s impedance decreases due to less pronounced polarization effects and a more consistent charge carrier mobility. This phenomenon is consistent with the findings reported in the literature [44,45,46,47,48]. The exponent values used in the analysis determined using Origin 8 software, help quantify this behavior and are illustrated in Fig. 9, which shows how temperature influences these parameters. Using Origin 8 software, Eq. (9) is employed to determine exponent values. The situation deteriorates with rising temperature, indicating that Non-overlapping Small Polaron Tunneling (NSPT), which is pertinent for researching conduction phenomena in the boundary of the alternating regime, is the most suitable conduction model [44]. This leads to the determination of the carrier’s binding energy Wm at that specific point. This can be discovered by using the following equation [49]:
The binding energy is denoted by Wm, and the Boltzmann constant is represented by KB. The exponent s becomes: in case the ratio of of \({W}_{m}\)/\({K}_{B}T\) is big.
The binding energy is determined by taking the linear slope of “s” and getting Wm = 0.183 eV. An Arrhenius-type characteristic is observed in the conductivity [50]:
Where T is the temperature, \({E}_{a}\) is the activation energy, A is the pre-exponential factor and \({K}_{B}\) is the Boltzmann constant (\({K}_{B}\) = 8.617 × 10−5 eV K−1). The temperature dependence of the conductivity Ln (\({\sigma }_{{dc}}\) T) vs 1000/T is illustrated in Fig. 10. The activation energy estimated from the slope of the linear fit is about 0.43 eV. Such value indicates that the conduction mechanism for the present system may be due to the polaron hopping based on electron carriers. In addition, it is evident that the values of the activation energy derived from conductivity and the frequency corresponding to the relaxation peaks of the imaginary part of the impedance (Z″) are different, which explains why the process of relation and the mechanism of conduction do not use the same charge carriers.
3.4 Dielectric and Modulus studies
The dielectric polarization and dielectric constants were assessed by looking at our sample’s dielectric characterization. Based on the mathematical framework introduced at [51], the electric modulus formalism considers both conduction and relaxation.
where the real and imaginary portions of the complex modulus are denoted by M′ and M″ correspondingly. They are articulated through the following relationships:
The frequency shift of the dielectric constant can be explained by the dispersion caused by Maxwell-Wagner interfacial polarization, according to Koop’s phenomenological theory. The real and imaginary components of the permittivity are denoted by the formulas ε = ε′ + j ε′′, which expresses the complex relative dielectric permittivity. The evolutions of ε′ versus frequency for the generated compound at different temperatures are shown in Fig. 11a. The real part of the permittivity ε′ has larger values at low frequencies because of the double-exchange interaction between the ferromagnetically coupled Fe3+ and Fe2+ ions. Since the real element of permittivity is related to stored energy, the charge begins to accumulate at the grain boundary as the temperature rises. Furthermore, it is evident that ε′ exhibits increased stability and increases with temperature. The evolution of ε′ is generally attributed to the four types of polarizations (atomic, electronic, dipolar, and interfacial) [52]. It has been proven that the evolution of ε′ with temperature is related, at low frequencies, to dipolar and interfacial polarizations [53].
As a result, the imaginary component of permittivity ε′′ for the compound as a function of frequency and temperature is shown in Fig. 11b. This figure clearly shows that the samples’ ε′′ declines with increasing frequency. In general, the imaginary component of permittivity is influenced by polarization and the conduction mechanism [50]. The dielectric constant is quite small because the dipoles follow the alternating field at higher frequencies [54]. Furthermore, the non-Debye behavior is supported by the absence of a relaxation peak at all temperatures. Furthermore, the frequency-dependent variation of M″ at different sample temperatures is shown in Fig. 12. It indicates that the value of M″ increases with increasing frequency and reaches its maximum (Mmax), reaffirming the presence of relaxation. Temperature-dependent relaxation processes in the material are shown by the shifting of the maximum value of the M″ (Mmax) peak to the higher frequency side. There may be a variation in capacitance as indicated by the magnitude of Mmax changing with temperature. The presence of a non-Debye-type [55] conduction mechanism in the material is suggested by asymmetric peak broadening, which also suggests relaxation spreading with a different time constant. The material Bi₀.₇Ba₀.₃(FeTi)₀.₅O3 demonstrates exceptional promise for a range of applications due to its unique combination of structural stability and electronic properties. Additionally, its high dielectric constant and low loss tangent render it suitable for use in high-frequency electronic devices and energy storage systems, such as capacitors and supercapacitors. The material’s enhanced electrical conductivity, resulting from Fe doping, further supports its utility in electronic components. Furthermore, the incorporation of Ba into the BiFeO₃ lattice is highlighted, as it plays a crucial role in modifying the dielectric properties, which could be leveraged in advanced sensor and actuator technologies.
4 A literature review and current study
To clearly present the added value of doping and the advancements achieved through our work, we have carefully compared the results obtained from BiFeO₃ with those from our doped sample, Bi0.7Ba0.3(FeTi)0.5O3, across several characterization techniques.
X-Ray Diffraction (XRD): Pure BiFeO₃: XRD patterns for BiFeO₃ typically show characteristic peaks at 2θ values around 32.6°, 39.3°, 46.6°, and 55.7°, which are indicative of the orthorhombic phase [56, 57]. The XRD results often reveal significant lattice distortions due to the inherent strain and phase stability issues. Doped Bi0.7Ba0.3(FeTi)0.5O3: The XRD analysis of the doped sample shows a clear stabilization of the perovskite structure in the rhombohedral phase. This stabilization reduces lattice distortions and improves phase purity. The doping process not only enhances the phase stability but also refines the crystal structure, which can lead to better material properties.
Williamson-Hall Analysis: For BiFeO₃, the Williamson-Hall plot indicates that strain is the predominant factor contributing to peak broadening, with a linear relationship suggesting significant lattice strain effects [58]. Doped Bi0.7Ba0.3(FeTi)0.5O3: In contrast, the Williamson-Hall analysis for the doped sample shows a more balanced contribution from size and strain. The reduced strain broadening observed in the doped sample indicates improved crystallinity and a more uniform microstructure, which is a direct benefit of the doping process.
Scanning Electron Microscopy (SEM) Images: SEM images of BiFeO₃ typically reveal a granular morphology with larger, irregularly sized grains, which can vary depending on synthesis conditions [59]. Doped Bi0.7Ba0.3(FeTi)0.5O3: The SEM images of the doped sample display a more homogeneous grain structure with smaller, more uniform grains. This refined microstructure is a result of the doping, which improves the material’s consistency and potentially its performance in practical applications.
Impedance Spectroscopy: Impedance spectroscopy results for BiFeO₃ show high dielectric constants with multiple semicircles in the impedance spectra, indicating contributions from both grain and grain boundary effects [60, 61]. The doped sample exhibits a more pronounced temperature and frequency dependence with lower impedance values, suggesting improved conduction properties and reduced grain boundary effects. This enhancement reflects better charge transport mechanisms due to doping.
AC Conductivity Analysis: The AC conductivity of BiFeO₃ is relatively low, showing an increase with temperature due to hopping mechanisms. Our sample demonstrates significantly higher AC conductivity, with a strong dependence on frequency and temperature [62, 63]. This improvement is indicative of enhanced charge carrier mobility and more efficient conductivity mechanisms facilitated by the doping.
Dielectric and Modulus Studies: BiFeO₃ typically shows a high dielectric constant with significant temperature dependence. The dielectric constant decreases with frequency due to relaxation mechanisms [64]. The modulus spectra often reveal prominent grain and grain boundary effects. The prepared sample Bi0.7Ba0.3(FeTi)0.5O3 exhibits an increased dielectric constant with a more stable behavior across temperature and frequency ranges. The reduced grain boundary effects and improved dielectric properties underscore the beneficial impact of doping.
5 Conclusions
The perovskite Bi0.7Ba0.3(FeTi)0.5O3 was synthesized via sol–gel synthesis and confirmed to be a single-phase sample with high density by room temperature X-ray diffraction, energy dispersive X-ray analysis, and scanning electron microscopy. We used impedance spectroscopy and modulus analysis to do a thorough examination of electrical properties, including conduction processes and temperature-dependent relaxation. Studies on modulus and impedance confirmed the compound’s non-Debye nature.
Furthermore, an electrical equivalent circuit (Rg + Rgb/CPEgb) was used to describe the semiconductor character that was linked to the grain and grain boundary contributions, according to impedance analysis. The real (ε′) and imaginary (ε″) components of permittivity behaved in a frequency-dependent manner during dielectric tests conducted at ambient temperature, suggesting the presence of a relaxation time distribution and the influence of charge carrier density. The electrical and dielectric properties that have been recently identified have enormous promise for new breakthroughs and enhanced efficiency in a variety of technical domains, including as electronics, telecommunications, and energy storage.
References
Eerenstein W, Mathur ND, Scott JF (2006) Multiferroic and magnetoelectric materials. Nature 442:759–765
Lan Y, Feng X, Zhang X, Shen Y, Wang D (2016) Effects of Bi doping on structural and magnetic properties of double perovskite oxides Sr2FeMoO6. Phys Lett A 380:2962–2967
Paul AK, Reehuis M, Ksenofontov V, Yan BH, Hoser A, Többens DM, Abdala PM, Adler P, Jansen M, Felser C (2013) Lattice instability and competing spin structures in the double perovskite insulator Sr2FeOsO6. Phys Rev Lett 111:1
Saxena M, Tanwar K, Maiti T (2017) Environmental friendly Sr2TiMoO6 double perovskite for high temperature thermoelectric applications. Scr Mater 130:205–209
Bhuyan S, Sivanand K, Panda SK, Kumar R, Hu J (2011) Resonance-based wireless energizing of piezoelectric components. IEEE Magn Lett 2:6000204
Slavney AH, Hu T, Lindenberg AM, Karunadasa HI (2016) A bismuth-halide double perovskite with long carrier recombination lifetime for photovoltaic applications. J Am Chem Soc 138:2138–2141
Gao X, Xu D, Du J, Li J, Yang Z, Sun J (2017) Controlled hydrothermal synthesis and optical properties of 3D flower-like CeO2 building with 3D hierarchical porous structure. J Mater Sci Mater Electron 28:17587–17591
Kumar MM, Palkar VR, Srinivas K, Suryanarayana SV (2000) Ferroelectricity in a pure BiFeO3 ceramic. Appl Phys Lett 76:2764–2766
Halder S, Bhuyan S, Das SN, Sahoo S, Choudhary RNP, Das P, Parida K (2017) Structural, morphological, dielectric and impedance spectroscopy of lead-free Bi(Zn2/3Ta1/3)O3 electronic material. Appl Phys A 123:781
Feng HJ, Liu FM (2008) First-principles prediction of coexistence of magnetism and ferroelectricity in rhombohedral Bi2FeTiO6. Phys Lett A 372:1904–1909
Rhazouani OE, Slassi A, Ziat Y, Benyoussef A (2017) Competing exchange couplings in Sr2CrReO6 double perovskite. Phys Lett A 381:1177–1180
Fresia E, Katz L, Ward R (1959) Cation substitution in perovskite-like phases 1,2. J Am Chem Soc 81:4783–4785
Viola MDC, Martinez-Lope M, Alonso J, Velasco P, Martinez J, Pedregosa J, Carbonio R, Fernández-Díaz M (2002) Induction of colossal magnetoresistance in the double perovskite Sr2CoMoO6. Chem Mater 14:812–818
Tayari F et al. (2024) Crystal Structural Characteristics and Electrical Properties of Novel Sol-Gel Synthesis of Ceramic Bi0. 75Ba0. 25 (FeMn)0.5O3. Materials 17:3797
Ritter C, Ibarra M, Morellon L, Blasco J, Garcia J, De Teresa J (2000) J Phys Condens Matter 12:8295
Dhanalakshmi B et al. (2016) Impedance spectroscopy and dielectric properties of multiferroic BiFeO3/Bi0.95Mn0.05FeO3–Ni0.5Zn0.5Fe2O4 composites. Ceram Int 42:2186–2197
Benamara M et al. (2023) Light-enhanced electrical behavior of a Au/Al-doped ZnO/p-Si/Al heterostructure: insights from impedance and current-voltage analysis. RSC Adv 13:28632–28641
Nassar KI et al. (2023) Exploring bismuth-doped polycrystalline ceramic Ba0.75Bi0.25Ni0.7Mn0.3O3: synthesis, structure, and electrical properties for advanced electronic applications. RSC Adv 13:24023–24030
Srivastava A, Garg A, Morrison FD (2009) Impedance spectroscopy studies on polycrystalline BiFeO3 thin films on Pt/Si substrates. J Appl Phys 105:054103
Purohit V, Choudhary RNP (2020) Structural, dielectric and electrical properties of BiFeO3 and BaTiO3 modified Bi(Mg0.5Ti0.5)O3. Mater Chem Phys 256:123732
Ghandouri AE et al. (2023) Effect of manganese doping on structural, optical, morphological, and dielectric properties of Ba (Ti1-x Mn x) O3 lead-free ceramics for energy storage in supercapacitors. Environmental Science and Pollution Research 1–17
Tayari F et al. (2024) Exploring Enhanced Structural and Dielectric Properties in Ag-Doped Sr (NiNb)0.5O3 Perovskite Ceramic for Advanced Energy Storage. Ceramics 7:958–974
Joshi S et al. (2024) Nd substitution response on structural, dielectric, and electrical features of bismuth iron titanate. Ceram Int 50:1643–1654
Elbasset A et al. (2017) Effect of Sr addition on piezoelectric properties and the transition temperature of BaTiO3. Glass Phys Chem 43:91–97
Mesrar M et al. (2021) Impedance spectroscopy and permittivity study of (1-x) NBT-xBT ceramics. Ceram–Silikáty 66:354–364
Wu J, Wang J (2009) N-derived signals in the x-ray photoelectron spectra of N-doped anatase TiO2. J Appl Phys 105:123535
Jonscher K, Husain A (1996) Dynamic transport on humid silica surface I. J Phys B Condens Matter 217:29–34
Identification of electrochemical processes by frequency response analyzer Solartron®- Claude Gabrielli—1998
Barsoukov E, Kim DH, Lee H-S, Lee H, Yakovleva M, Gao Y, Engel JF (2003) Comparison of kinetic properties of LiCoO2 and LiTi0.05Mg0.05Ni0.7Co0.2O2 by impedance spectroscopy. J Solid State Ion 161:19–29
Barsoukov E, Macdonald JR. Electrochemical impedance: theory, experiment, and applications. (2005).
Singh L, Won Kim I, Sin BC, Woo SK, Hyun SH, Mandal KD, Lee Y (2015) Combustion synthesis of nano-crystalline Bi2/3Cu3Ti2.90Fe0.10O12 using inexpensive TiO2 raw material and its dielectric characterization. Powder Technol 280:256–265
Chatterjee S, Mahapatra PK, Choudhary RNP, Thakur AK (2004) Complex impedance studies of sodium pyrotungstate—Na2W2O7. Status Solidi A 201:588–595
Omri A, Bejar M, Es-Souni M, Valente MA, Graça MPF, Costa LC (2012) Electrical conductivity and dielectric analysis of La0.75(Ca,Sr)0.25Mn0.85Ga0.15O3 perovskite compound. J Alloy Compd 536:173–178
Costa MM, Pires J´unior GFM, Sombra ASB (2010) Dielectric and impedance properties’ studies of the of lead doped (PbO)-Co2Y type hexaferrite (Ba2Co2Fe12O22(Co2Y) Mater Chem Phys 123:35–39
Cole KS, Cole RH (1941) Chem Phys B 41:9
D Johnson, Z-view, Impedance Software, Version 2.1a, Scribner Associates Inc, (1990–1998)
Cordoba-Torres P, Mesquita TJ, Devos O, Tribollet B, Roche V, Nogueira RP (2012) On the intrinsic coupling between constant-phase element parameters α and Q in electrochemical impedance spectroscopy. Electro Acta 72:172–178
Hirschorn B, Orazema ME, Tribollet B, Vivier V, Frateur I, Musiani M (2010) Determination of effective capacitance and film thickness from constant-phase-element parameters. Electro Acta 55:6218–6
Lily KK, Prasad K, Choudhary RNP (2008) Impedance spectroscopy of (Na0.5Bi0.5)(Zr0.25Ti0.75)O3 lead-free ceramic. J Alloy Compd 453:325–331
Chihaoui N, Bejar M, Dhahri E, Valente MA, Costa LC, Graça MPF (2015) Int J Mater Eng Technol 13:129
James R, Parkash C, Prasad G(2006) Structural properties and impedance spectroscopy of excimer laser ablated Zr substituted BaTiO3 thin films J Phys D Appl Phys 39:1635–1641
Lanfredi S, Saia PS, Lebullenger R, Hernandes AC (2002) Electric conductivity and relaxation in fluoride, fluorophosphate and phosphate glasses: analysis by impedance spectroscopy. Solid State Ion 146:329–339
Jonscher AK (1996) Universal Relaxation Law. Chelsea Dielectric Press, London
Ghosh A (1990) Frequency-dependent conductivity in bismuth-vanadate glassy semiconductors. Phys Rev B 41:1479
Kalinin SV et al. (2002) Potential and impedance imaging of polycrystalline BiFeO3 ceramics. J Am Ceram Soc 85:3011–3017
Migahed MD et al. (1996) Dielectric relaxation and electric modulus behavior in poly (vinyl alcohol)‐based composite systems. J Appl Polym Sci 59:655–662
Ali I et al. (2014) Role of grain boundaries in the conduction of Eu–Ni substituted Y-type hexaferrites. J Magn Magn Mater 362:115–121
Kolodziej H, Sobczyk L (1971) Investigation of the dielectric properties of potassium hydrogen maleate. Acta Phys Pol A 39:59
Qian X et al. (2001) Impedance study of (PEO) 10LiClO4–Al2O3 composite polymer electrolyte with blocking electrodes. Electrochim acta 46:1829–1836
Mohamed M, Bakr, Hui Wang, Fuess H (2010) Dielectric relaxation and magnetic properties of Cr doped GaFeO3. J Phys D: Appl Phys 43:455409
Assoudi N et al. (2018) Physical properties of Ag/Ca doped Lantanium manganite. J Mater Sci: Mater Electron 29:20113–20121
Jones JR, Hench LL (2001) Biomedical materials for the new millennium: perspective on the future. Mater Sci Technol 17:891–900
Martirena HT, Burfoot JC (1974) Grain-size and pressure effects on the dielectric and piezoelectric properties of hot-pressed PZT-5. Ferroelectrics 7:151–152
Pattanayak S et al. (2013) Impedance spectroscopy of Gd-doped BiFeO 3 multiferroics. Appl Phys A 112:387–395
Ranjan R et al. (2011) Impedance and electric modulus analysis of Sm-modified Pb (Zr0. 55Ti0. 45) 1− x/4O3 ceramics. J Alloy Compd 509:6388–6394
Liu K et al. (2011) Structural, electronic and optical properties of BiFeO3 studied by first-principles. J Alloy Compd 509:1901–1905
Sen A et al. (2020) Influence of Ba and Mo co-doping on the structural, electrical, magnetic and optical properties of BiFeO3 ceramics. Mater Res Express 7:016312
Bai X. Size and doping effect on the structure, transitions and optical properties of multiferroic BiFeO3 particles for photocatalytic applications. Diss. Université Paris Saclay (COmUE), 2016.
Zhou Y et al. (2018) Statistical analysis of moisture’s effect on AC breakdown strength of TiO2 nanofluids. J Mol Liq 249:420–428
Elansary M et al. (2024) Preparation of CoZrxFe2–xO4 magnetic nanoparticles: In-depth investigation of Zr (IV) effect on photocatalytic performance, magnetic and electrochemical properties. Mater Today Commun 40:109838
Tayari F et al. (2023) Structural, morphology, Raman spectroscopy, magnetic and electrical proprieties of BaNi0. 5Mn0. 25Fe0. 25O3 ceramic for electronic applications. Indian J Phys 97:3545–3555
Nassar KI et al. (2024) Structural, electrical properties of bismuth and niobium-doped LaNiO3 perovskite obtained by sol–gel route for future electronic device applications. Indian Journal of Physics 1–9
Sharif MK et al. (2016) Synthesis and characterization of Zr and Mg doped BiFeO3 nanocrystalline multiferroics via micro emulsion route. J Alloys Compd 667:329–340
Ahmad J et al. (2021) A facile strategy for the preparation of bismuth ferrite nanoparticles: influence of calcination temperature on structural, dielectric, and morphological characteristics. Colloids Surf A: Physicochemical Eng Asp 628:127328
Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through a Large Research Project under grant number RGP2/201/45.
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
Faouzia Tayari: Conceptualization, Methodology, Formal Analysis, Investigation, Writing—Original Draft. Silvia Soreto Teixeira: Data Curation, Software, Validation, Writing—Review & Editing. M.P.F. Graça: Supervision, Resources, Project Administration, Funding Acquisition. Manel Essid: Visualization, Investigation, Resources. Kais Iben Nassar: Conceptualization, Methodology, Supervision, Writing—Review & Editing, Correspondence.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tayari, F., Teixeira, S.S., Graça, M.P.F. et al. Investigating structural, dielectric, and electrical characteristics of sol–gel synthesized perovskite ceramic Bi0.7Ba0.3(FeTi)0.5O3. J Sol-Gel Sci Technol (2024). https://doi.org/10.1007/s10971-024-06549-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10971-024-06549-9