Abstract
In this research, we successfully synthesized magnesium aluminate (MgAl2O4) spinel nanoparticles using a sol-gel process, with stearic acid serving as a capping agent. The synthesis process involved calcination at 900 °C for 4 h, resulting in the formation of nanoparticles with an average crystallite size of approximately 12 nm, as determined through Debye–Scherrer analysis and X-ray diffraction (XRD) data. The optical band gap was measured as 2.84 eV using Diffuse Reflectance Spectroscopy (DRS) analysis. Additionally, we found the mean pore size of the nanoparticles to be 20.2 nm through Brunauer–Emmett–Teller (BET) analysis. We characterized the resulting powders using various techniques, including Fourier Transform Infrared (FTIR) spectroscopy, Field Emission Scanning Electron Microscopy (FESEM), Energy-Dispersive X-ray Spectroscopy (EDS), and Vibrating Sample Magnetometry (VSM). We conducted electrochemical investigations utilizing the Chronopotentiometry (CP) technique. The electrochemical analysis demonstrated that MgAl2O4 spinel nanoparticles exhibit a noteworthy hydrogen storage capacity of 4000 mAh/g, highlighting their potential as promising candidates for hydrogen storage applications. This comprehensive study underscores the successful synthesis, thorough characterization, and exceptional electrochemical performance of MgAl2O4 spinel nanoparticles, firmly positioning them as valuable materials for advancing hydrogen storage technologies.
Graphical Abstract

Highlight
-
MgAl2O4 spinel nanoparticles were synthesized using the gel stearic acid method.
-
The structural properties were studied by various microscopic and electrochemical methods.
-
MgAl2O4 spinel nanoparticles showed an excellent hydrogen storage capacity of 4000 mAh/g.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The rapid growth of fossil fuel consumption and the increase in human society have led to a rise in environmental pollution and heightened concerns about the future of the planet [1]. Furthermore, the amount and type of energy used plays a significant role in daily human activities and the future. Therefore, one safe and effective way to reduce environmental pollution is to utilize green energies, renewable sources, and clean fuels like hydrogen [2]. To achieve this goal, research is currently being conducted on the production of hydrogen from renewable energy sources [3]. Hydrogen can serve as an energy carrier due to its characteristics, such as renewability, high energy content, and efficient energy conversion [4]. Hydrogen has the potential for use in various industries, including steel production, hybrid cars, engine fuel, and fuel cells [5, 6]. Notably, hydrogen is the lightest and most abundant element in the universe. When compared to other fossil fuels, such as gasoline, hydrogen boasts a higher energy density by volume and is typically stored in large tanks [7]. Hydrogen must be stored efficiently, safely, and cost-effectively. Hydrogen can be stored both chemically and physically. Storing hydrogen in nanomaterials is often based on physical absorption [8]. Several types of materials have been used to store hydrogen, such as transition mixed metal oxides [9], polymers [10], metal-organic frameworks (MOFs) [11], and graphene nanocomposites [12]. Nanoparticles are superior to other materials for hydrogen storage due to their high surface-to-volume ratio, structural stability during physicochemical reactions, small size for absorbing and releasing hydrogen molecules, and reversible storage potential [13]. Hydrogen storage in solid-state materials is one of the safest and most effective methods for storage [14].
So far, many spinel oxides have been synthesized by different methods like, NiCr2O4 [15], NiAl2O4 [16], and MgCr2O4 [17]. Previous reports have proven that spinels, such as BaAl2O4 and CoAl2O4 have been used in hydrogen storage [18, 19]. Among the spinels, magnesium aluminate spinel (MgAl2O4) has been the focus of researchers due to its excellent physicochemical properties, such as electrochemical, dielectric, thermal, mechanical, and optical properties. MgAl2O4 has been extensively studied in various forms, like nanocomposites and nanoparticles, for applications in energy storage [20,21,22]. Furthermore, when MgAl2O4 is combined with other materials, like metal oxides, it leads to the formation of new nanostructures that have the potential to be applied in hydrogen storage [23]. One of the best advantages of magnesium aluminate oxide compared to other materials for hydrogen storage is its high specific surface area and porous structure, which results in the formation of numerous active sites for hydrogen absorption [24]. Magnesium aluminate has a broad range of applications such as hydrogen production [25], humidity sensors [26], catalysts [27], and supercapacitors [21]. MgAl2O4 has been synthesized using various methods, including sol-gel [28], Co-precipitation [29], hydrothermal [27], solution combustion [30], and solid-state [31].
The sol-gel method for preparing magnesium aluminate spinel (MgAl2O4) offers distinct advantages over alternative methods. This method ensures high purity, employing pure metallic precursors and minimizing impurities in the final material. Additionally, sol-gel-derived MgAl2O4 typically requires lower sintering temperatures, which enhances energy efficiency and preserves material properties. The technique also allows for precise dopant control, enabling uniform incorporation of ions to tailor material properties for specific applications [15, 32, 33].
In this work, MgAl2O4 spinel nanoparticles were synthesized via a sol-gel process, using stearic acid as a capping agent for the first time. Stearic Acid, with its long carbon chain, effectively prevents nanoparticle agglomeration. Magnesium aluminate was investigated by different techniques such as XRD, FTIR, FESEM, EDS, DRS, VSM, and BET. Hydrogen storage capacity and various parameters like copper sheet surface, cycle number, and current intensity were studied. The results revealed that MgAl2O4 nanoparticles could be a promising material for hydrogen storage.
2 Experimental
2.1 Materials and methods
Aluminum nitrate (Al(NO3)3·9H2O), magnesium acetate (with better solubility and more stability) (Mg(OAc)2·4H2O), and stearic acid (C18H36O2) with 99.9% purity were purchased from Merck. The XRD patterns of MgAl2O4 nanoparticles were analyzed by a Model PTS 3003 SEIFERT diffractometer using Cu Kα radiation (λ = 1.54 Å) and in the 2θ range from 10° to 80° to study the structural development and crystallization of the sample. The FTIR spectrum of the MgAl2O4 nanoparticles was recorded with an MB100 (BOMEM) spectrophotometer using a KBr pellet. FESEM was used to investigate the size distribution and surface morphology of the samples (JEOL-64000, Japan). The energy dispersive spectrometry (EDS) evaluation was performed by Philips EM208. To study the magnetic properties of the sample, a Vibrating Sample Magnetometer was used (Meghnatis Daghigh Kavir Co., Kashan, Iran). The band gap of the sample was determined through UV absorption spectra (Shimadzu UV/3101 PC) within a wavelength range of 300 to 500 nm. Brunauer–Emmett–Teller (BET) specific surface areas of the catalysts were determined through N2 adsorption/desorption tests performed on an ASAP-2010 analyzer (Micromeritics, USA). The chronopotentiometry method was applied to estimate the discharge capacity (hydrogen storage capacity) of a sample using the SAMA 500 electro-analyzer system in Iran (potentiostat/galvanostat).
2.2 Preparation of MgAl2O4 spinel Nanoparticles
MgAl2O4 spinel nanoparticles (NPs) were prepared via the sol-gel process, using Magnesium acetate, Aluminum nitrate as the cation source, and stearic acid as a capping agent. First, 10 mmol stearic acid was melted in a beaker at 73 °C. Then, 1 mmol magnesium acetate and 2 mmol aluminum nitrate were dissolved in distilled water (pH = 4). The solutions containing metallic ions were added to stearic acid and stirred at a temperature of 65–85 °C to form a viscous gel. After cooling the gel at room temperature, it was heated in an electric oven at 85 °C for 24 h to dry. During this time, metal cations diffusion from the aqueous phase to the organic phase, resulting in a homogeneous sol. Finally, the dried gel was calcined at temperatures of 700 and 900 °C for 4 h to obtain MgAl2O4 spinel nanoparticles. Scheme 1. displays a schematic diagram of synthesized MgAl2O4 spinel NPs at temperatures of 700 and 900 °C for 4 h (Table 1).
2.3 Electrochemical hydrogen storage
The chronopotentiometry method is a significant technique for estimating the hydrogen storage capacitance. In this electrochemical cell, Ag/AgCl, Pt, and Cu-MgAl2O4 are the reference, counter (anode), and working (cathode or coated-copper plate) electrodes, respectively. The electrolyte is 6 M KOH aqueous solution. In this system, a current intensity (±1 mA) is applied between the counter (anode) and working (cathode) electrodes, and the potential differences are estimated between the working and the Ag/AgCl (reference) electrodes. To fabricate an electrode of Cu-MgAl2O4, the copper sheet used as a thin substrate for the Cu/MgAl2O4. The MgAl2O4 powder is sonicated in ethanol for 10 min. A pure copper sheet (1 × 1 cm2) is coated by a substrate of MgAl2O4 powder at 100 °C [13].
3 Results and discussion
3.1 XRD analysis
XRD diffractograms of MgAl2O4 spinel nanoparticles calcined at temperatures of 700 and 900 °C for 4 h in the 2θ range from 10º–80º are illustrated in Fig. 1a. As can be seen in Fig. 1a, the XRD pattern of MgAl2O4 calcined at 700 °C demonstrated weak peaks with low intensity. Therefore, it can be concluded that the primary structure of these nanoparticles is being formed, and crystals have not yet grown completely at 700 °C. An increase in temperature up to 900 °C leads to an increase in the size of the crystals, resulting in broadened diffraction peaks. All diffraction peaks at 2θ values of 19º, 31º, 37º, 45º, 56º, 59.6º and 65.5º can be indexed (111), (220), (311), (400), (422), (511), and (440) crystal planes, respectively. The results exhibited that MgAl2O4 (JCPDS- 01-071-2499) has a cubic crystal structure with space group Fd-3m and lattice parameters a = b = c = 8.05Å. No detected impurities like MgO or Al2O3. The average crystallite size of the MgAl2O4 spinel nanoparticles calcined at 900 °C for 4 h was calculated from the XRD diffractogram utilizing the Debye–Scherrer relation (Eq. 1) [34]:
Where β is the width of the XRD peak at half height, k is a shape factor of about 0.9 for spherical-shaped nanoparticles, D is the mean particle diameter, λ is the wavelength (0.15418 nm), and θ is the diffraction angle. The average crystallite size was calculated utilizing the linear fit of Eq. 1 to the plot cosθ vs 1/β depicted in Fig. 1b. The average crystal size was estimated to be 12 nm (increasing temperature = promoting crystal growth) [35]. The Debye−Scherrer equation calculates the crystallite size without considering lattice distortion and micro-strain induced in the structure. Therefore, the Williamson-Hall (W-H) relation was used to analyze the effect of lattice on strain the peak broadening [36, 37] (Eq. 2):
Where ε is the strain induced in the lattice, and D is the average crystallite size. According to Fig. 1c, the lattice strain and crystallite size have been calculated using the Williamson-Hall plot. Figure 1c displays the fit of Eq. 2 to the β cos θ vs 4 sin θ plot. The average crystallite size and lattice strain were estimated by determining the intercept and slope of the graph, these were found to be 17.35 nm and 296 × 10−5, respectively. The crystallite size obtained by the Williamson-Hall equation is slightly larger than that calculated by the Debay-Scherrer equation. This difference may be due to considering the strain impact as calculated by the Williamson-Hall equation [38] (Fig. 2).
3.2 FT-IR spectroscopy
The results of FT-IR spectroscopy for MgAl2O4 spinel NPs at 900 °C are shown in Fig. 2. Two absorption bonds can be seen at 522–697 cm−1, which are attributed to the vibrations involving metal-oxygen bondssuch as Al-O stretching in the AlO6 group and lattice vibration of Mg-O stretching [28, 39]. The vibration bond observed at 1630 cm−1 is related to bending (H-O-H), and the vibration band around 3440 cm−1 corresponds to the OH group [40].
3.3 FESEM and energy dispersive X-ray (EDS)
The surface morphology of the MgAl2O4 spinel NPs calcined at 900 °C was studied using FESEM analysis. The FESEM images of the sample are displayed in Fig. 3a, b. The powder sample showed the formation of uniform spherical shapes and homogeneity of the structure. Furthermore, the FESEM images of the MgAl2O4 spinel nanoparticles was analyzed using the ImageJ software [39]. The histogram plot of the sample is shown in Fig. 3c. The average grain size was obtained 25 nm. The EDX spectrum of the MgAl2O4 spinel nanoparticles is depicted in Fig. 4. The EDS results demonstrate the presence of oxygen, aluminum, and magnesium elements without impurity. The elementary constituents of MgAl2O4 spinel NPs are displayed in Table 1 [41, 42].
3.4 Optical properties
The energy gap (Eg) and absorption coefficient are desirable features of semiconductors that determine their applications in optoelectronics. The results of the UV–vis absorption spectrum of MgAl2O4 spinel NPs in the wavelength range of 300–500 nm are depicted in Fig. 5a, b. The absorption peak was observed at 380 nm due possibly to the O2−→ Al3+ charge transition [43]. The photon energy (Eg) has been obtained utilizing Tuac’s relation (Eg) (Eq. 3).
Where h is the Planck’s constant (6.62607004 × 10−34 m2kg/s), ʋ is the Frequency (Hz), α is the Absorption coefficient, A is the Energy independent constant, Eg band gap energy (eV) and n is the nature of transmission. Accordingly, the band gap energy of the nanoparticle was evaluated using a graph of (αhν)2 values against the band gap energy (hν) axis extrapolating the linear portion of the absorption edge to find the interruption by energy axis. Figure 5b shows the optical band gap of MgAl2O4 spinel nanoparticles. The value of the direct band gap for MgAl2O4 spinel nanoparticles came out to be 2.84 eV [31]. Previous reports showed that the optical reflectance of MgَAl2O4 spinel nanoparticles depends on the calcination temperature [44]. As the calcination temperature is increased beyond 800 °C, the crystallite size of MgAl2O4 increases leading to a decrease in the energy of the band gap. In other words, the optical reflectance properties of MgAl2O4 spinel NPs can be attributed to its cubic crystal structure system. The reduction in bandgap energy may be due to the accumulation of defect states between the valence and conduction bands [45]. Consequently, MgAl2O4 spinel NPs can be employed as semiconductor and photocatalyst.
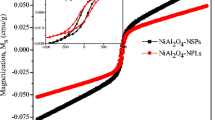
3.5 VSM studies
The magnetic behavior of MgAl2O4 spinel NPs has been measured (Fig. 6). The magnetic properties of the sample were observed at room temperature. Exploring the M-H curve confirms the ferromagnetic properties of MgAl2O4 spinel NPs. The ferromagnetic property of MgAl2O4 spinel NPs has already been reported [46]. The saturation magnetization (Ms), remanence magnetization (Mr), and coercivity field (Hc) were about 0.0154 emu/g, 0.0244 emu/g, and 200 Oe, respectively. The remanence ratio (Mr/Ms) value was estimated at around 1.584. The saturation magnetization (Ms) of 0.0154 emu/g indicates their capacity for strong magnetization under an external field. This Mr value suggests notable magnetic memory after the field is removed, while the Hc of 200 Oe signifies MgAl2O4 resistance to demagnetization. The value of 1.584 highlights the (Mr/Ms) ability to maintain magnetization, showcasing the potential for applications in data storage and magnetic devices. Normal spinel, also known as cubic spinel, can exhibit ferromagnetic behavior due to the presence of magnetic ions in its crystal structure. This mineral’s chemical formula is AB2O4, with A and B representing different metal cations. Ferromagnetism emerges from the alignment of magnetic moments of these cations within the crystal lattice. When magnetic ions occupy both the A and B sites, they interact via exchange interactions that favor parallel alignment of their magnetic moments. When a magnetic field is applied, the magnetic moments align themselves with the field, leading to saturation magnetization. Bulk normal spinel may contain magnetic domains, with groups of atomic magnetic moments aligning in the same direction. The specific combination of magnetic ions and their arrangement in the lattice, as well as temperature, determine whether ferromagnetism is observed in each normal spinel compound. Ferromagnetism in the normal spinel is a result of quantum mechanical interactions and the behavior of magnetic moments in the crystal structure.
3.6 BET technique
The specific surface region of the MgAl2O4 spinel NPs at 900 °C was estimated using a BET- BJH technique. The result is illustrated in Fig. 7a, b. The N2 adsorption/desorption and the categorization of IUPAC demonstrate a characterization of Class IV in the adsorption isotherms with hysteresis loops [35]. The MgAl2O4 spinel NPs (which have a mesoporous structure) exhibit hysteresis of H3-type. The specific surface area of 108.1 m²/g indicates a substantial surface area available for interactions with hydrogen molecules, suggesting a high adsorption capacity. The pore volume of 0.5459 cm³/g is noteworthy, as it implies that the material can hold a significant amount of hydrogen gas, making it suitable for various practical applications, including hydrogen fuel cells and transportation. Furthermore, the mean pore size of 20.2 nm provides insights into the material’s pore structure, which is vital for hydrogen mobility and accessibility. The diversity in pore sizes within the nanoparticles allows for accommodating different masses of hydrogen, facilitating their diffusion into and out of the material. Overall, the BET results emphasize the material’s potential for gas adsorption and storage with a particular focus on hydrogen, thanks to the significant specific surface area, pore volume, and mean pore size. On the other hand, the FESEM results provide visual confirmation of the nanoparticles’ size, shape, and uniformity, which can be important for applications that rely on a consistent and well-defined nanoparticle structure.
3.7 Hydrogen storage capacity
According to Fig. 8, the discharge capacity of copper foam without MgAl2O4 attendance is almost equal to 3 mAh/g. Figure 9 illustrates the discharge properties of the Cu- MgAl2O4 electrode after 5 cycles under a constant current of 1 mA. Additionally, this diagram indicates that placing the Cu-MgAl2O4 electrode in an alkaline medium can affect its capacity. Electrochemical hydrogen absorption mechanisms occur during three reactions: Volmer, Tafel, and Heyrovsky [47]. The electrolyte solution was prepared using a 6 M KOH combination dissolved in deionized water (Proton source). As a result of the decomposition of water, H atoms are formed (Eq. 4). During the charging process (Volmer reaction), the electrolyte is separated around the working electrode, and hydrogen is adsorbed on the MgAl2O4 nanoparticles surface (Eq. 5).
According to the Volmer, the reaction reduction of H2O to hydroxyl ions and the adsorption of hydrogen atoms onto the working electrode surface; result in the formation of subsurface hydrogen (Hss).
Then, subsurface hydrogen atoms (Hss) diffuse as bulk-absorbed hydrogen (Habs).
The increase of surface accumulated hydrogen causes the migration of adsorbed H (Hads) into the MgAl2O4 network. During the discharge process, which occurs in the opposite direction to the charging process, the absorbed hydrogen atoms are desorbed from the surface of the working electrode and turned back into water, releasing an electron. The adsorption of hydrogen atoms on the surface of the Cu-MgAl2O4 (cathode) is a type of physical adsorption [48]. The discharge capacity enhanced from 2380 mAh/g in the first cycle to 4000 mAh/g after 5 cycles. The increase in discharge capacity can likely be explained by the formation of more active sites for hydrogen desorption/absorption on the surface of the working electrode, the pore distribution, and the surface-to-volume ratio of MgAl2O4 spinel nanoparticles. [49]. Based on the physical adsorption equations for electrochemical hydrogen storage, such as the sidewise Tafel reaction (Eqs. 9, 10) and the Heyrovsky process (Eq. 11), if the hydrogen absorption energy is less than the released energy, gaseous hydrogen (H2) is formed.
Figure 10 shows the cycling performance of the MgAl2O4 nanoparticles at a constant current of 1 mA. The amount of stored hydrogen in the working electrode can be measured by the discharge capacity. The storage capacity (SC) can be estimated from the charge/discharge curves of the electrodes according to Eq. 11 [50].
The MgAl2O4 spinel nanoparticles are suitable due to their fewer cycle number, low cost, and desirable electrochemical discharge capacity. Table 2 displays a comparison between MgAl2O4 spinel nanoparticles and the previously reported nanomaterials.
4 Conclusion
In summary, MgAl2O4 spinel nanoparticles (NPs) were successfully synthesized through the sol-gel process at a temperature of 900 °C, utilizing stearic acid as a capping agent. The obtained results revealed an average crystallite size of approximately 12 nm and a specific surface area of 108.1 m2.g−1, both of which were associated with a mesoporous structure. The EDS and FESEM analyses confirmed the purity of the acquired MgAl2O4 spinel NPs, exhibiting a lack of impurities and showcasing a consistently small, uniform, and spherical morphology.
Furthermore, the optical band gap, calculated as 2.84 eV using Diffuse Reflectance Spectroscopy (DRS), falls within the range indicative of efficient photo-catalytic behavior. The Vibrating Sample Magnetometer (VSM) analysis indicated ferromagnetic behaviors within the nanoparticles.
Owing to their distinctive structure and properties, MgAl2O4 spinel nanoparticles hold promise for application in hydrogen energy storage. Notably, the nanoparticles demonstrated a noteworthy maximum discharge capacity of 4000 mAh/g, solidifying their potential as a suitable candidate for hydrogen storage applications.
References
Bilen K, Ozyurt O, Bakırcı K, Karslı S, Erdogan S, Yılmaz M, Comaklı O (2008) Energy production, consumption, and environmental pollution for sustainable development: a case study in Turkey. Renew Sustain Energy Rev 12:1529–1561. https://doi.org/10.1016/j.rser.2007.03.003
Sharma S, Agarwal S, Jain A (2021) Significance of hydrogen as economic and environmentally friendly fuel. Energies 14:7389. https://doi.org/10.3390/en14217389
Qureshi F, Yusuf M, Kamyab H, Zaidi S, Junaid Khalil M, Arham Khan M, Azad Alam M, Masood F, Bazli L, Chelliapan S, Abdullah B (2022) Current trends in hydrogen production, storage and applications in India: a review. Sustain Energy Technol Assessments 53:102677. https://doi.org/10.1016/j.seta.2022.102677
Orhan MF, Babu BS (2015) Investigation of an integrated hydrogen production system based on nuclear and renewable energy sources: Comparative evaluation of hydrogen production options with a regenerative fuel cell system. Energy 88:801–820. https://doi.org/10.1016/j.energy.2015.06.009
Liu W, Zuo H, Wang J, Xue Q, Ren B, Yang F (2021) The production and application of hydrogen in steel industry. Int J Hydrogen Energy 46:10548–10569. https://doi.org/10.1016/j.ijhydene.2020.12.123
Bartolucci L, Cennamo E, Cordiner S, Mulone V, Pasqualini F, Boot MA (2023) Digital twin of a hydrogen fuel cell hybrid electric vehicle: effect of the control strategy on energy efficiency. Int J Hydrogen Energy 48:20971–20985. https://doi.org/10.1016/j.ijhydene.2022.11.283
Tashie-Lewis BC, Nnabuife SG (2021) Hydrogen production, distribution, storage and power conversion in a hydrogen economy—a technology review. Chem Eng J Adv 8:100172. https://doi.org/10.1016/j.ceja.2021.100172
Chen, Y. P., Bashir, S., & Liu, J. L. (2017). Nanostructured materials for next-generation energy storage and conversion. Springer Berlin, Heidelberg, pp117–142. https://doi.org/10.1007/978-3-662-53514-1
Eslami A, Lachini SA, Shaterian M, Karami M, Enhessari M (2023) Synthesis, characterization, and hydrogen storage capacity of MgMn2O4 spinel nanostructures. Inorg Chem Commun 110875. https://doi.org/10.1016/j.inoche.2023.110875
Mahato N, Jang H, Dhyani A, Cho S (2020) Recent progress in conducting polymers for hydrogen storage and fuel cell applications. Polymers (Basel) 12:2480. https://doi.org/10.3390/polym12112480
Cao Y, Dhahad HA, Zare SG, Farouk N, Anqi AE, Issakhov A, Raise A (2021) Potential application of metal-organic frameworks (MOFs) for hydrogen storage: Simulation by artificial intelligent techniques. Int J Hydrogen Energy 46:36336–36347. https://doi.org/10.1016/j.ijhydene.2021.08.167
Deniz CU, Mert H, Baykasoglu C (2021) Li-doped fullerene pillared graphene nanocomposites for enhancing hydrogen storage: a computational study. Comput Mater Sci 186:110023. https://doi.org/10.1016/j.commatsci.2020.110023
Gholami T, Salavati-Niasari M, Varshoy S (2017) Electrochemical hydrogen storage capacity and optical properties of NiAl2O4/NiO nanocomposite synthesized by green method. Int J Hydrogen Energy 42:5235–5245. https://doi.org/10.1016/j.ijhydene.2016.10.132
Boateng E, Chen A (2020) Recent advances in nanomaterial-based solid-state hydrogen storage. Mater Today Adv 6:100022. https://doi.org/10.1016/j.mtadv.2019.100022
Javed M, Khan AA, Kazmi J, Mohamed MA, Khan MN, Hussain M, Bilkees R (2021) Dielectric relaxation and small polaron hopping transport in sol-gel-derived NiCr2O4 spinel chromite. Mater Res Bull 138:111242. https://doi.org/10.1016/j.materresbull.2021.111242
Iqbal Y, Shah WH, Khan B, Javed M, Ullah H, Khan N, Khan AR, Asghar G, Safeen A (2023) Small polaron hopping transport mechanism, dielectric relaxation and electrical conduction in NiAl2O4 electro-ceramic spinel oxide. Phys Scr 98:065951. https://doi.org/10.1088/1402-4896/acd5ba
Javed M, Khan AA, Ahmed MS, Khisro SN, Kazmi J, Bilkees R, Khan MN, Mohamed MA (2020) Temperature dependent impedance spectroscopy and electrical transport mechanism in sol-gel derived MgCr2O4 spinel oxide. Phys B Condens Matter 599:412377. https://doi.org/10.1016/j.physb.2020.412377
Gholami T, Salavati-Niasari M, Varshoy S (2016) Investigation of the electrochemical hydrogen storage and photocatalytic properties of CoAl2O4 pigment: Green synthesis and characterization. Int J Hydrogen Energy 41:9418–9426. https://doi.org/10.1016/j.ijhydene.2016.03.144
Salehabadi A, Salavati-Niasari M, Sarrami F, Karton A (2017) Sol-Gel auto-combustion synthesis and physicochemical properties of BaAl2O4 nanoparticles; electrochemical hydrogen storage performance and density functional theory. Renew Energy 114:1419–1426. https://doi.org/10.1016/j.renene.2017.07.119
Ganesh I (2013) A review on magnesium aluminate (MgAl2O4) spinel: synthesis, processing and applications. Int Mater Rev 58:63–112. https://doi.org/10.1179/1743280412Y.0000000001
Alam MW, Kumar VGD, Ravikumar CR, Prashantha SC, Murthy HCA, Kumar MRA (2022) Chromium (III) doped polycrystalline MgAl2O4 nanoparticles for photocatalytic and supercapacitor applications. J Phys Chem Solids 161:110491. https://doi.org/10.1016/j.jpcs.2021.110491
Ullah F, Qureshi MT, Sultana K, Saleem M, Al Elaimi M, Abdel Hameed R, ul Haq S, Ismail HS, Anwar MS (2021) Structural and dielectric studies of MgAl2O4–TiO2 composites for energy storage applications. Ceram Int 47:30665–30670. https://doi.org/10.1016/j.ceramint.2021.07.244
Zeng D-W, Peng S, Chen C, Zeng J-M, Zhang S, Zhang H-Y, Xiao R (2016) Nanostructured Fe2O3/MgAl2O4 material prepared by colloidal crystal templated sol–gel method for chemical looping with hydrogen storage. Int J Hydrogen Energy 41:22711–22721. https://doi.org/10.1016/j.ijhydene.2016.09.180
Wang F, Luo M, Liu Q, Shao C, Yang Z, Liu X, Guo J (2023) Preparation of Pt/MgAl2O4 decalin dehydrogenation catalyst for chemical hydrogen storage application. Catal Lett 1–15. https://doi.org/10.1007/s10562-023-04283-5
Gómez-Solís C, Peralta-Arriaga SL, Torres-Martínez LM, Juárez-Ramírez I, Díaz-Torres LA (2017) Photocatalytic activity of MAl2O4 (M = Mg, Sr and Ba) for hydrogen production. Fuel 188:197–204. https://doi.org/10.1016/j.fuel.2016.10.038
Das S, Rahman ML, Mondal PP, Mahapatra PL, Saha D (2021) Screen-printed MgAl2O4 semi-thick film based highly sensitive and stable capacitive humidity sensor. Ceram Int 47:33515–33524. https://doi.org/10.1016/j.ceramint.2021.08.260
Zhang X (2009) Hydrothermal synthesis and catalytic performance of high-surface-area mesoporous nanocrystallite MgAl2O4 as catalyst support. Mater Chem Phys 116:415–420. https://doi.org/10.1016/j.matchemphys.2009.04.012
Milani SS, Kakroudi MG, Vafa NP, Rahro S, Behboudi F (2021) Synthesis and characterization of MgAl2O4 spinel precursor sol prepared by inorganic salts. Ceram Int 47:4813–4819. https://doi.org/10.1016/j.ceramint.2020.10.051
Nam S, Lee M, Kim B-N, Lee Y, Kang S (2017) Morphology controlled Co-precipitation method for nano structured transparent MgAl2O4. Ceram Int 43:15352–15359. https://doi.org/10.1016/j.ceramint.2017.08.075
Ghosh SR, Mukherjee S, Banerjee S (2020) Development of spinel magnesium aluminate by solution combustion route using thiourea and urea as. Fuel J Eng Res [TJER] 17:135–141. https://journals.squ.edu.om/index.php/tjer/article/view/3680
Mukherjee S (2022) Development of spinel magnesium aluminate by modified solid state process and its characterization. Mater Today Proc 67:314–319. https://doi.org/10.1016/j.matpr.2022.07.113
Bai N, Liu X, Li Z, Ke X, Zhang K, Wu Q (2021) High-efficiency TiO2/ZnO nanocomposites photocatalysts by sol–gel and hydrothermal methods. J Sol-Gel Sci Technol 99:92–100. https://doi.org/10.1007/s10971-021-05552-8
Khan AA, Javed M, Rauf Khan A, Iqbal Y, Majeed A, Hussain SZ, Durrani SK (2017) Influence of preparation method on structural, optical and magnetic properties of nickel ferrite nanoparticles. Mater Sci 35:58–65. https://doi.org/10.1515/msp-2017-0006
Enhessari M, Lachini SA (2019) CuMn2O4 nanostructures: facial synthesis, structural, magnetical, electrical characterization and activation energy calculation. Int J Bio Inorg Hybr Nanomater 8:39–45
Sanjabi S, Obeydavi A (2015) Synthesis and characterization of nanocrystalline MgAl2O4 spinel via modified sol–gel method. J Alloys Compd 645:535–540
Akbar N, Javed M, Arif Khan A, Masood A, Ahmed N, Mehmood RY, Khisro SN, Abdul MAS, Mohammad Haniff MAS, Shah A (2023) Zircon-type CaCrO4 chromite nanoparticles: synthesis, characterization, and photocatalytic application for sunlight-induced degradation of rhodamine B. ACS Omega 8:30095–30108. https://doi.org/10.1021/acsomega.3c02457
Javed M, Khan AA, Akbar N, Kazmi J, Dar A, Mohamed MA (2023) Low-temperature dielectric relaxation mechanism and correlated barrier hopping transport in neodymium perovskite chromite. Mater Res Bull 165:112303. https://doi.org/10.1016/j.materresbull.2023.112303
Javed M, Akbar N, Khan AA, Alsubhe E, Mohammad Alghamdi S, Karamti H, Albeydani OA, ben Ahmed S, Kazmi J, Khisro SN, Mohamed MA (2023) Photocatalytic activity of sol-gel self-combustion derived MCr2O4 (M= Mg, Ni) spinel chromites for photodegradation of organic dyes. Mater Today Commun 35:105716. https://doi.org/10.1016/j.mtcomm.2023.105716
Li F, Zhao Y, Liu Y, Hao Y, Liu R, Zhao D (2011) Solution combustion synthesis and visible light-induced photocatalytic activity of mixed amorphous and crystalline MgAl2O4 nanopowders. Chem Eng J 173:750–759. https://doi.org/10.1016/j.cej.2011.08.043
Banerjee S, Mukherjee S, Ghosh SR (2021) Evaluation of properties of non-stoichometric alumina magnesia spinel using thiourea as fuel by varying soaking time. J Eng Res [TJER] 18:44–51. https://doi.org/10.53540/tjer.vol18iss1pp44-51
Javed M, Khan AA, Khisro SN, Majeed A, Kazmi J, Bilkees R, Hussain M, Mohamed MA (2022) Charge conduction mechanism and non-debye type relaxation in LaCrO3 perovskite orthochromite. Mater Chem Phys 290:126522. https://doi.org/10.1016/j.matchemphys.2022.126522
Javed M, Arif Khan A, Kazmi J, Akbar N, Ahmed N, Khisro SN, Mohamed MA (2023) Investigation on electrical transport and dielectric relaxation mechanism in TbCrO3 perovskite orthochromite. J Alloys Compd 955:170181. https://doi.org/10.1016/j.jallcom.2023.170181
Nassar MY, Ahmed IS, Samir I (2014) A novel synthetic route for magnesium aluminate (MgAl2O4) nanoparticles using sol–gel auto combustion method and their photocatalytic properties, Spectrochim. Acta A Mol Biomol Spectrosc 131:329–334. https://doi.org/10.1016/j.saa.2014.04.040
Ewais EMM, El-Amir AAM, Besisa DHA, Esmat M, El-Anadouli BEH (2017) Synthesis of nanocrystalline MgO/MgAl2O4 spinel powders from industrial wastes. J Alloys Compd 691:822–833. https://doi.org/10.1016/j.jallcom.2016.08.279
Goswami B, Rani N, Vats R, Bhukkal C, Ahlawat R (2021) Highly crystalline and narrow bandgap MgAl2O4: synthesis and characterization. In: AIP Conference Proceedings, AIP Publishing, p 020045. https://doi.org/10.1063/5.0052473
Abbasi Asl E, Haghighi M, Talati A (2020) Enhanced simulated sunlight-driven magnetic MgAl2O4-AC nanophotocatalyst for efficient degradation of organic dyes. Sep Purif Technol 251:117003. https://doi.org/10.1016/j.seppur.2020.117003
Kaur M, Pal K (2019) Review on hydrogen storage materials and methods from an electrochemical viewpoint. J Energy Storage 23:234–249. https://doi.org/10.1016/j.est.2019.03.020
Gholami T, Pirsaheb M (2021) Review on effective parameters in electrochemical hydrogen storage. Int J Hydrogen Energy 46:783–795. https://doi.org/10.1016/j.ijhydene.2020.10.003
Sangsefidi FS, Salavati-Niasari M (2017) Thermal decomposition synthesis, characterization and electrochemical hydrogen storage characteristics of Co3O4–CeO2 porous nanocomposite. Int J Hydrogen Energy 42:20071–20081. https://doi.org/10.1016/j.ijhydene.2017.05.127
Salehabadi A, Salavati-Niasari M, Gholami T (2018) Green and facial combustion synthesis of Sr3Al2O6 nanostructures; a potential electrochemical hydrogen storage material. J Clean Prod 171:1–9. https://doi.org/10.1016/j.jclepro.2017.09.250
Samimi F, Ghiyasiyan-Arani M, Salavati-Niasari M (2022) Synthesis of calcium manganese oxide with different constructions as potential materials for electrochemical hydrogen storage. Fuel 321:124074. https://doi.org/10.1016/j.fuel.2022.124074
Zhu H, Cao Y, Zhang J, Zhang W, Xu Y, Guo J, Yang W, Liu J (2016) One-step preparation of graphene nanosheets via ball milling of graphite and the application in lithium-ion batteries. J Mater Sci 51:3675–3683. https://doi.org/10.1007/s10853-015-9655-z
Acknowledgements
The authors of this article would like to express their gratitude and appreciation for the financial support provided by the Mazandaran University Research Council and Freie Universitat Berlin.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
In accordance with the guidelines set by the “Journal of Sol-Gel Science and Technology” we hereby provide a detailed account of the contributions made by each author to the research presented in the manuscript titled “Sol-Gel Synthesis, Characterization, and Electrochemical Evaluation of Magnesium Aluminate Spinel Nanoparticles for High-Capacity Hydrogen Storage.” ME: conceptualization, methodology, supervision conceived the research idea and formulated the objectives of the study. Designed the experimental methodology for the sol-gel synthesis of magnesium aluminate spinel nanoparticles. Provided oversight and guidance throughout the research project. SAL: synthesis and characterization conducted the experimental synthesis of magnesium aluminate spinel nanoparticles using the sol-gel method. Carried out detailed characterization of the synthesized nanoparticles using techniques such as X-ray diffraction (XRD) and scanning electron microscopy (SEM). Analyzed the characterization data and contributed to the interpretation of the results. MS: electrochemical evaluation designed and executed the electrochemical evaluation experiments to assess the hydrogen storage capacity of the synthesized nanoparticles. Conducted cyclic voltammetry and chronoamperometry experiments and collected relevant electrochemical data. Contributed to the discussion and analysis of the electrochemical results in the context of hydrogen storage applications. MK: data analysis and interpretation compiled and organized the experimental data obtained from the synthesis, characterization, and electrochemical evaluation. Conducted statistical analysis of the data and interpreted the trends observed. Collaborated with other authors to correlate the results with the theoretical framework and the broader context of hydrogen storage materials. AE: manuscript preparation and writing drafted the initial version of the manuscript, incorporating the contributions from all authors. Ensured the manuscript adheres to the journal’s formatting and citation guidelines. Integrated feedback from co-authors and revised the manuscript for clarity, coherence, and scientific accuracy. All authors have read and approved the final version of the manuscript submitted to the “Journal of Sol-Gel Science and Technology.” We affirm that the work presented represents a collaborative effort where each author’s expertise contributed significantly to different aspects of the research process.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eslami, A., Lachini, S.A., Shaterian, M. et al. Sol-gel synthesis, characterization, and electrochemical evaluation of magnesium aluminate spinel nanoparticles for high-capacity hydrogen storage. J Sol-Gel Sci Technol 109, 215–225 (2024). https://doi.org/10.1007/s10971-023-06260-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06260-1