Abstract
Lead halide perovskite materials have outstanding optical properties such as color purity and efficient fluorescence in the visible spectrum. However, the toxicity of lead makes the synthesis, processing, use and disposal of such materials hazardous for humans and the environment. Therefore, the exploration of different perovskite materials containing the transition metal Mn(II) and fluoride anions might lead to more appealing alternatives towards more sustainable and environmentally friendly functional materials. Nanocrystals primarily consisting of cubic CsMnF3 – a polymorph so far only reported at pressures of 3 × 104 bar – were synthesized from manganese(II) acetate and caesium fluoride precursors using a heating-up approach in high boiling organic solvents. The concentration of defects could be tuned by changing the amount of fluorinating precursor CsF, with more defects produced when an excess of CsF was used in the synthesis. The structure, morphology, optical and magnetic properties of the product nanoparticles were studied. Due to their X-ray sensitized fluorescence, likely caused by defects in the crystal structure, these materials are promising for potential applications in dosimetry.
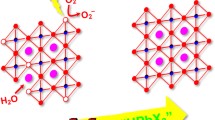
Graphical Abstract
CsMnF3 nanocrystals were synthesized by a heating-up method using stoichiometric or excess quantities of the fluoride precursor CsF to control the concentration of defects (CMF and CMF-d). The obtained nanoparticles exhibited X-ray sensitized luminescence, enhanced by a factor of up to 3.75 after irradiation with X-rays for 2 h.

Highlights
-
Synthesis of cubic CsMnF3 nanocrystals at ambient pressure demonstrated (previously only reported as hexagonal at ambient pressure).
-
HF-free bottom-up synthesis of CsMnF3 nanocrystals in high boiling organic solvents.
-
Defect-related luminescence of CsMnF3 nanocrystals, sensitized by X-ray irradiation.
-
A Néel temperature of 60 K was measured for cubic CsMnF3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Crystalline nanomaterials with a cubic perovskite structure of the type ABX3, with corner sharing octahedra of [BX6]4− around A+-cations often show optical properties of different kinds which are relevant for a range of fields [1,2,3]. For example, the oxide perovskite barium titanate (BaTiO3) is a material used in non-linear optics due to its strong second harmonic generation [4]. Caesium-lead based materials where the X-anion site is occupied by a halide, most frequently Cl− and Br−, have band gaps allowing for the emission of visible light when excited at higher energy, for instance in the UV [5]. Many studies have investigated the formation and optical properties of such nanomaterials and details about their history and applications can be found in the literature [6].
Though lead halide perovskites possess outstanding luminescence properties, lead is a historically well-known toxin for humans [7]. Therefore, several experimental and theoretical studies aiming to find materials with similar properties, but containing more benign constituents, have been carried out [8,9,10,11,12]. Additionally to the aim of avoiding the use of highly toxic elements, the study of different or unknown compounds can potentially lead to the discovery of useful properties or property combinations, compared with CsPbX3 (X = Cl−, Br−) [13].
Recently, an algorithm designed to predict the structure of halide-perovskite materials has been proposed [14]. In their study, Pilania and co-authors correctly predicted the crystal structures of many less studied perovskite materials. However, in some cases their model fails, such as for CsMnF3, which they predict as a cubic perovskite, while CsMnF3 was shown to display a hexagonal crystal structure at ambient conditions [15]. Using the Goldschmidt tolerance factor, determined by an equation proposed as early as 1926, which can be used to predict crystal structures based on the ionic radii of their constituents, CsMnF3 is correctly predicted as hexagonal [16, 17]. However, in their study Pilania et al. included several parameters besides the Goldschmidt tolerance factor for predicting structures, such as the octahedral factor, bond valence radii, electronegativity, and orbital radii, leading to their distinct result [14]. CsMnF3 crystallizes in a hexagonal P 63/mmc structure at ambient conditions and the cubic Pm-3m perovskite structure of CsMnF3 has so far only been observed at elevated pressure above 3 × 104 bar [18, 19]. The cubic CsMnF3 nanoparticles stable at ambient conditions presented here exhibit fluorescence, showing photoluminescence emissions similar to other defect-rich fluorides as well as X-ray sensitized luminescence, which was studied using time-resolved spectroscopy [20,21,22,23,24,25]. Due to this property, CsMnF3 nanoparticles could find application in dosimetry. Known materials for dosimetry applications include sulfates such as CaSO4:Dy or the fluorides LiF:Mg,Cu,P and KMgF3:Eu [26]. Defect-rich CsMnF3 could represent another promising nanomaterial for this type of application [27, 28]. Like in the case of cubic KMnF3 and hexagonal CsMnF3, cubic CsMnF3 exhibits antiferromagnetic ordering of magnetic spins at temperature below its Néel temperature. This can be observed in temperature dependent magnetic susceptibility measurements [29,30,31]. Moreover, their magnetic properties suggest that they could be used as a model system alongside hexagonal CsMnF3 to study the effects of the crystal structure on magnetic and optical properties. Together with other relevant fluoroperovskites such as KMnF3 and RbMnF3, CsMnF3 could be used to study optical and magnetic properties and their interactions [32,33,34]. Therefore, cubic CsMnF3 may represent a promising compound for the study of fundamental properties of fluoroperovskites.
To our knowledge CsMnF3 has not been reported in its cubic polymorph at ambient pressure before. Here we report the synthesis of two samples of cubic CsMnF3 nanocrystals stable at ambient conditions called CMF and CMF-d. CMF-d represents a more defect-rich version of CMF. Both samples were obtained via a bottom-up synthesis in organic solvents at 160 °C using manganese(II) acetate and caesium fluoride as the starting materials, with CMF-d being synthesized in the presence of a three-fold excess of caesium fluoride. We also report the decomposition product Cs2H3F5 which was obtained when CMF-d was kept at the reaction temperature of 160 °C for 24 h instead of 1. The structure and composition of the products were studied using X-ray diffraction (XRD), transmission electron microscopy (TEM) and energy dispersive X-ray spectroscopy (EDX).
2 Materials and methods
Manganese(II)acetate 98% (Sigma-Aldrich, Buchs), octadecene 90% (Acros, Pittsburgh), oleic acid 90% (Sigma-Aldrich, Buchs), caesium fluoride 99.9% (Sigma-Aldrich, Buchs) were used as received without further purification. Moreover, the standard laboratory technical solvents methanol, ethanol, acetone, and hexane were used as obtained from various suppliers.
2.1 Synthesis of CMF
A solution of manganese acetate (86.9 mg, 0.5 mmol) in methanol (3 mL) was prepared, which was added to a mixture of octadecene (7.5 mL) and oleic acid (7.5 mL). The resulting mixture was heated to 120 °C under N2 flux for 30 min to form a transparent dark red solution. After cooling to 33 °C a solution of caesium fluoride (229.0 mg, 1.5 mmol) in methanol (1.5 mL) was added. Upon the addition of the caesium fluoride solution the reaction mixture turned light yellow and then faintly pink. After stirring at room temperature for 30 min, the reaction mixture was heated to 120 °C for 50 min, to evaporate the methanol. The temperature was subsequently raised to 160 °C. After 60 min the heating was stopped, and the reaction mixture washed after cooling down naturally. Acetone (30 mL) was added to the reaction mixture before collecting the solid product by centrifugation (4000 rpm, 10 min). The supernatant was discarded and the solid redispersed in hexane (10 mL) by sonicating (10 min). Acetone (35 mL) was added before centrifuging (4000 rpm, 10 min). This step was repeated three times. The last centrifugation step was extended to 30 min. The resulting light-yellow product named CMF was dried at room temperature and analyzed (99.8 mg, 81%).
2.2 Synthesis of CMF-d and Cs2H3F5
Manganese acetate (87.4 mg, 0.5 mmol) dissolved in methanol (3 mL) was added to a mixture of octadecene (7.5 mL) and oleic acid (7.5 mL). The resulting mixture was heated to 120 °C under N2 flux for 30 min to form a transparent dark red solution. After cooling to 33 °C caesium fluoride (455.8 mg, 3 mmol) in methanol (3 mL) was added. Upon the addition of the caesium fluoride solution the reaction mixture turned light yellow and then faintly pink. After stirring at room temperature for 35 min, the reaction mixture was heated to 120 °C for 50 min, to remove the methanol. The temperature was subsequently raised to 160 °C. After 60 min half (7.5 mL) of the reaction mixture was removed from the reaction vessel and washed. Acetone (40 mL) was added to the reaction mixture before collecting the solid product by centrifugation (4000 rpm, 10 min). The supernatant was discarded and the solid redispersed in hexane (10 mL) by sonicating (10 min). Acetone (35 mL) was added before centrifuging (4000 rpm, 10 min). This step was repeated for a total of three times. The resulting light-yellow product was named CMF-d and was dried at room temperature and analyzed (103.3 mg, apparent yield 168%). The second half of the reaction mixture was washed after 24 h of reaction time by centrifuging for 30 min, redispersing in hexane (5 mL) and centrifuging again for 30 min. The resulting solid of Cs2H3F5 was dried at room temperature (9.2 mg, 3%).
Though the formation of fluorinated side-products was not directly observed, it cannot be fully excluded. Since CsF is also used in fluorination reactions of organic compounds, the fluorination of the solvent should be always carefully considered [35].
Powder X-ray diffraction (XRD) was measured on a PANalytical Xpert Pro diffractometer using copper Kα radiation. Samples were prepared with a flat surface on a zero-background sample holder and measured with a step size of 0.0668451 °θ, the time per step was 140.335 s. To determine the crystallite size of a Cs2H3F5 sample the Scherrer equation was used [36, 37]. A reference of LaB6 was measured to determine the peak broadening associated with the instrumentation. Then the peak positions and full-width-half-maxima of the [111], [200] and [220] were used to obtain three values for the crystallite size. The final value reported here is the average of these three values.
Transmission electron microscopy (TEM) and scanning transmission electron microscopy (STEM) images were recorded on a Thermo Scientific Talos F200X operated at 200 kV. TEM samples were prepared by dropping particle dispersions on copper grids covered with a lacey amorphous carbon foil. Energy-dispersive X-ray spectroscopy (EDX) was performed on the same TEM instrument using the SuperX integrated EDX-system with four silicon drift detectors.
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were carried out on a Mettler Toledo TGA/DSC 3+ system using alumina crucibles.
UV-visible absorption spectroscopy was carried out on a Jasco V-660 UV-visible spectrometer equipped with an integrating sphere to measure solid samples in reflection. In line absorption was measured on the same instrument, without the integrating sphere.
Photoluminescence spectra (PL) was measured on a Jasco FP-8500 fluorometer equipped with a solid sample holder. Spectra were not corrected to remove reflections originating from the measurement geometry, however MgO was measured as a non-luminescent reference, and it was found that no significant contributions arose from reflections in the sample chamber. Time resolved spectroscopy was measured on the same instrument, fixing both emission and excitation wavelengths; data points were collected every second. To measure X-ray sensitization, a sample was irradiated with copper k-α radiation generated by the X-ray tube of an X-Pert Pro diffractometer for 2 h. Photoluminescence was then measured in the solid state using a JASCO FP-8500 fluorometer.
Magnetic susceptibility was studied using an MPMS3 device (QuantumDesign). The sample was measured for 1 s per datapoint without any bias field with an excitation amplitude of 10 Oe at 83 Hz.
3 Results and discussion
3.1 Synthesis
Two samples of CsMnF3 nanoparticles named CMF and CMF-d were prepared by heating the precursors to 160 °C for one hour in the organic solvents octadecene and oleic acid. The difference in the synthetic conditions between the two samples was the quantity of CsF used in the fluorination step: CMF was obtained using a 1:3 molar ratio, while a 1:6 molar ratio between manganese(II)acetate and CsF was used for CMF-d (Fig. 1a, b). The decomposition product Cs2H3F5 was obtained when a CMF-d synthesis was heated to 160 °C for a longer time i.e., 24 h (Fig. 1b) and Supplementary Fig. S1) likely due to the dissolution of the initial product under these conditions. This hypothesis is also supported by the low yield of 3% in which Cs2H3F5 was obtained [38].
3.2 Structure and morphology
CMF and CMF-d were studied with respect to their crystal structure using XRD. Their morphology was also evaluated using TEM. Both products primarily consisted of cubic CMF, however an impurity most probably consisting of Cs2H3F5 could be revealed by XRD-analysis which was more pronounced in CMF-d (Fig. 2). Similarly to the cubic CsMnF3-nanoparticles reported here, ZrO2-nanoparticles have been obtained in their non-thermodynamically stable polymorph in a bottom-up synthesis, showing that non-thermodynamically stable crystal structures can form at ambient conditions when such synthetic methods are employed [39]. CsMnF3 has previously been reported in its hexagonal crystal structure at room temperature and atmospheric pressure while its cubic polymorph has been previously observed only at elevated pressure above 3 × 104bar [15, 18, 19]. To study the stability and processability of the products described here, their thermal evolution was investigated using TGA and DSC. The results of this analysis are displayed in Figure 3a–c and show that none of the products underwent significant weight changes in air up to 200 °C. All products showed an exothermic peak between 200 and 300 °C when heated in air, indicating a possible oxidation or partial oxidation reaction, the exact nature of which could not be inferred because relevant literature data to compare the results to are scarce.
TEM investigations were carried out to study the crystal structure and morphology of the products obtained. Lattice fringes of CMF and CMF-d nanoparticles measuring around 3–5 nm could be clearly observed (Fig. 3d, e), while Cs2H3F5 decomposed extremely rapidly under the electron beam, preventing the acquisition of high-resolution TEM images. However, a bright field TEM image of Cs2H3F5 is displayed in Fig. 3f), in which smooth particle edges indicate a high degree of crystallinity as also suggested by the sharp peaks of the XRD diffractogram. The Scherrer equation was used to determine the crystallite size of Cs2H3F5 to be 70 nm, which is in good agreement with the particle size observed in TEM. The crystallite size of CMF and CMF-d could not be studied using the Scherrer equation, since the diffraction peaks originating from the Cs2H3F5 impurity overlap with the CsMnF3 peaks and therefore reliable full width at half maximum determination could not be carried out. Nonetheless, the peak broadening observed agrees with the small crystallites imaged using TEM.
The elemental composition of CMF and CMF-d was further investigated using EDX (Fig. 4a, b). As expected, CMF contains caesium, manganese, and fluorine in the ratio of 1.13:1:3 (Table 1 and Fig. 4a) and Supplementary Fig. S1). This agrees well with the structural information gained from XRD: the main component of the product is CsMnF3 with an impurity of Cs2H3F5 reflected in the stoichiometric excess of caesium.
a BF-TEM and EDX analysis for CMF, b BF-TEM and EDX analysis for CMF-d. For EDX spectra corresponding to a and b see Supplementary Fig. S2
CMF-d was obtained using twice the stoichiometrically required amount of the fluorinating agent CsF and the yield of the solid product collected was 168% with respect to the initial quantity of Mn(II) precursor. This suggests that though no crystalline phases besides cubic CsMnF3 and traces of Cs2H3F5 were observed in XRD (Fig. 2), a portion of the product was probably amorphous, leading to the unusually high yield. This lies in contrast to CMF, which was synthesized with a stoichiometric quantity of CsF and had a yield of 81% with respect to Mn(II) added to the reaction mixture. Moreover, the composition of both CMF and CMF-d was mapped using EDX (Fig. 4a, b). In the case of CMF, caesium, manganese, and fluorine are contained within the same area, while for CMF-d caesium and fluorine spread beyond the area containing manganese. Furthermore, the elemental quantities measured vary across different areas of the CMF-d sample, showing a slight excess of caesium in some areas and caesium deficiency with respect to manganese in others (Supplementary Figs. S3–S5). Therefore, it can be inferred that in CMF-d the proportion of the impurity and amorphous matter is larger than in CMF and thus that CMF-d represents a less well-defined version of the CsMnF3 nanomaterial compared to CMF.
The products CMF and CMF-d have a cubic crystal structure with varying amounts of Cs2H3F5 impurity and consist of small nanoparticles of around 4 nm, while the degradation product Cs2H3F5 consisted of larger particles of around 50–100 nm. The reaction yield of CMF-d which exceeded 100%, together with the EDX analysis where caesium and fluorine spread beyond the area containing manganese, suggest that compared to CMF, CMF-d might contain other, likely amorphous, Mn-poor phases which are not revealed by XRD.
3.3 Optical properties
The optical absorption and emission spectra of the two materials were measured using UV-visible absorption and photoluminescence spectroscopy on dry powder samples.
Both CMF and CMF-d show a yellow coloration (Fig. 5), indicating a significant absorption in the blue region of the visible spectrum. While CMF shows broad peaks in its absorption spectrum, the absorption of CMF-d shows peaks compatible with the absorption bands reported elsewhere for KMnF3 synthesized in a hydrothermal process and KMgF3:Mn (0.5%) prepared in a thermally activated solid state synthesis [40, 41]. KMnF3 is a useful material to compare our samples to, because due to its cubic perovskite structure identical to that observed for CsMnF3 it contains the same [MnF6]4- octahedra surrounding the cations.
Some comparison could be drawn with the KMnF3 materials described by Sheng et al., which were also studied with respect to their photoluminescence. Emission bands were observed at around 414, 437 and 464 nm when exciting KMnF3 microparticles of different sizes and morphologies at 373 nm [40]. The relative intensities of the emissions were found to depend on the size and morphology of the particles. The PL spectra recorded for CMF and CMF-d also show a broad feature between 400 and 600 nm which is clearly composed of multiple bands. The maxima of the emission peaks for CMF and CMF-d are found at 468.5 and 464.5 nm (Fig. 6a, b), which coincides with one of the less intense band reported at 464 nm by Sheng and co-workers [40].
a Photoluminescence emission (PL) and excitation (PLE) spectra of CMF; the inset shows powder samples under 366 nm irradiation, b emission and excitation spectra CMF-d; the inset shows the powder under 366 nm irradiation, c emission spectra of CMF and CMF-d before (solid lines) and after (dashed lines) X-ray irradiation, d luminescence decay of CMF-d under constant 374 nm excitation
Similarly, an emission at 465 nm attributed to F3 centers has also been observed by Riley et al. in cubic KMgF3 single crystals [42]. An F-center is an anion vacancy filled with an electron and can lead to colorations in otherwise colorless materials. F2 and F3-centers are the combination of two and three F-centers, respectively, to form clusters [43].
Since the nature of defects causing fluorescence is relatively difficult to investigate, computational studies can help to identify the various defects leading to emissions. Such a study has been carried out by Modak and Modak who computationally describe defect bands in KMnF3 using hybrid DFT calculations and assign the aforementioned band at 465 nm to a potassium vacancy combined with a fluoride vacancy [44]. Their different result compared to the assignment of the same band to an F3 center by Riley et al. underlines the complexity of rationalizing defect-related emissions in fluoride perovskite materials. Modak and Modak assign further bands observed in KMnF3 at 566, 530, 440, 340 and 480 nm to one magnesium vacancy combined with two fluoride vacancies and to F, F2 and F3 centers, respectively.
The different intensity ratios of the emission bands observed in CMF and CMF-d samples compared to the ones reported for cubic KMnF3 and KMgF3 could be potentially accounted for by the difference in crystal size and the synthetic pathways chosen to prepare them [40]. In fact, the KMnF3 particles measured several micrometers in size and although their crystallite size was not reported, it can be assumed to be larger than the crystallite size of the CMF and CMF-d particles discussed here. This might mean that the crystallite surface to volume ratio is much higher in CMF and CMF-d. Because of the nature of the fluorescence, which likely originates from imperfections in the crystal structure, the synthetic pathway chosen and the changed surface to volume ratio could also alter the relative concentration of different types of defects such as surface and bulk defects in the material and therefore its PL emission profile.
The emission spectrum of CMF-d shows a much higher overall intensity than the emission spectrum of CMF (Fig. 6c) solid lines). This could be due to the higher concentration of defects in the CMF-d sample. Mn(II) emission is expected around 590 nm and thus cannot be responsible for the emission observed in CMF and CMF-d [45, 46]. Song et al. have studied the emission of Mn(II) doped KMgF3 cubic perovskite samples and report concentration quenching of the characteristic Mn(II) emission at 590 nm when Mn was present in the sample at concentrations above 20%, which might well explain the lack of an Mn(II) emission band in CMF and CMF-d [46].
Highly defect-rich materials with their complex energy transfer mechanisms are potential candidates for dosimetry applications [47]. Therefore, our samples were preliminarily investigated for their potential as high energy radiation sensing materials. Both our samples exhibited X-ray sensitized luminescence, with an increase in emission intensity of 3.75 and 1.5 times for CMF and CMF-d, respectively, after copper Kα X-ray irradiation for two hours (Fig. 6c). To study this further, the fluorescence decay of CMF-d was measured under continuous wave irradiation at 374 nm over 2 h (Fig. 6d). A double exponential was used to fit the luminescence decay of X-ray irradiated CMF-d (Fig. 6d), Eq. (1) (equation for double exponential fit) and Table 2.
The relaxation times obtained were τ1 = 194 s and τ2 = 5022 s. This indicates a luminescence decay behavior including more than one type of emitting center. Moreover, different emission bands are overlapping and respond differently to the X-ray irradiation (Fig. 6c), making the deconvolution of the emission centers particularly challenging.
CMF and CMF-d have been studied with respect to their optical properties using UV and PL spectroscopy. It was found that CMF-d exhibits more intense luminescence than CMF, both before and after X-ray irradiation suggesting that the emission in both samples originates from defects in the crystal structure. Considering these results, the defect structure of the materials studied strongly depends on the synthetic parameters chosen and materials with desired properties can be obtained by choosing a suitable synthesis.
3.4 Magnetic properties
Measuring the magnetic susceptibility of a material as a function of temperature gives information about properties such as the Néel temperature, above which an antiferromagnetic material becomes paramagnetic. Here, we measured the magnetic susceptibility of CMF only, considered to be the most structurally defined version of cubic CsMnF3. The cubic polymorph CMF shows antiferromagnetic ordering below 60 K (Fig. 7), between the values reported for hexagonal CsMnF3 and cubic KMnF3. Hexagonal CsMnF3 is known as an antiferromagnetic material below its Néel temperature at 53.5 K, while cubic KMnF3 has a Néel temperature at 88 K and cubic RbMnF3 at 83 K [30, 31, 48, 49]. An increase of magnetic susceptibility was observed below 30 K peaking at 7 K. Similar results have been reported in the literature for KMnF3 and have been attributed to uncompensated surface spins at the edge of nanocrystallites [50]. This explanation is consistent with the nanometric morphology of the CMF sample described here.
4 Conclusion
We report the synthesis of cubic CsMnF3 nanoparticles in organic solvents avoiding the use of HF. While CsMnF3 has only been reported in its hexagonal crystal structure at ambient conditions so far, the synthesis presented here leads primarily to the formation of cubic CsMnF3 nanoparticles. The materials obtained showed similar optical properties to KMnF3 which has been reported to exhibit defect-related fluorescence [40]. Therefore, this study provides an additional example of a fluoride material showing defect-related luminescence and some insight into how this luminescence can be tuned by changing the reaction conditions. In the future, thermoluminescence experiments could further elucidate the nature of such emissions in CsMnF3. Promising X-ray energy storage properties, possibly suitable for dosimetry applications, were shown by the X-ray sensitization of the fluorescence of the more defect-rich CsMnF3-sample. However, further studies about the X-ray sensitization and its proportionality in CsMnF3 are necessary to evaluate its suitability for these applications. Moreover, CsMnF3 exhibited antiferromagnetic ordering at temperatures below 60 K.
References
Hong K, Le QVan, Kim SY, Jang HW (2018) Low-dimensional halide perovskites: review and issues. J Mater Chem C 6:2189–2209. https://doi.org/10.1039/c7tc05658c
Wei Y, Cheng Z, Lin J (2019) An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem Soc Rev 48:310–350. https://doi.org/10.1039/c8cs00740c
Jena AK, Kulkarni A, Miyasaka T (2019) Halide perovskite photovoltaics: background, status, and future prospects. Chem Rev 119:3036–3103. https://doi.org/10.1021/acs.chemrev.8b00539
Karvounis A, Timpu F, Vogler‐Neuling VV et al. (2020) Barium titanate nanostructures and thin films for photonics. Adv Opt Mater 8:2001249. https://doi.org/10.1002/adom.202001249
Mao X, Sun L, Wu T et al. (2018) First-principles screening of all-inorganic lead-free ABX 3 perovskites. J Phys Chem C 122:7670–7675. https://doi.org/10.1021/acs.jpcc.8b02448
Akkerman QA, Rainò G, Kovalenko MV, Manna L (2018) Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat Mater 17:394–405. https://doi.org/10.1038/s41563-018-0018-4
Gidlow DA (2004) Lead toxicity. Occup Med 54:76–81. https://doi.org/10.1093/occmed/kqh019
Abfalterer A, Shamsi J, Kubicki DJ et al. (2020) Colloidal synthesis and optical properties of perovskite-inspired cesium zirconium halide nanocrystals. ACS Mater Lett 2:1644–1652. https://doi.org/10.1021/acsmaterialslett.0c00393
Kong D, Cheng D, Wang X et al. (2020) Solution processed lead-free cesium titanium halide perovskites and their structural, thermal and optical characteristics. J Mater Chem C 8:1591–1597. https://doi.org/10.1039/c9tc05711k
Liu S, Bin Y, Chen J et al. (2020) Efficient thermally activated delayed fluorescence from all- inorganic cesium zirconium halide perovskite nanocrystals. Angew Chem Int Ed 59:21925–21929
Xiong G, Yuan L, Jin Y et al. (2020) Aliovalent doping and surface grafting enable efficient and stable lead-free blue-emitting perovskite derivative. Adv Opt Mater 8:2000779. https://doi.org/10.1002/adom.202000779
Mao X, Sun L, Wu T et al. (2018) First-principles screening of all-inorganic lead-free ABX 3 perovskites. J Phys Chem C 122:7670–7675. https://doi.org/10.1021/acs.jpcc.8b02448
Wolf NR, Connor BA, Slavney AH, Karunadasa HI (2021) Doubling the stakes stakes: the promise of halide double perovskites doubling the stakes. Angew Chem Int Ed 60:2–17.
Pilania G, Balachandran PV, Kim C, Lookman T (2016) Finding new perovskite halides via machine learning. Front Mater 3:1–7. https://doi.org/10.3389/fmats.2016.00019
Zalkin A, Lee K, Templeton DH (1962) Crystal structure of CsMnF 3. J Chem Phys 37:697–699. https://doi.org/10.1063/1.1733149
Goldschmidt VM (1926) Die Gesetze der Krystallochemie. Sci Nat 14:477–485. https://doi.org/10.1007/BF01507527
Dubrovin RM, Pisarev RV (2020) Spontaneous magnetodielectric effect and its coupling to the lattice dynamics in fluoroperovskites. J Exp Theor Phys 131:189–200. https://doi.org/10.1134/S1063776120070043
Syono Y, Akimoto S, Kohn K (1969) Structure relations of hexagonal perovskite-like compounds ABX 3 at high pressure. J Phys Soc Jpn 26:993–999. https://doi.org/10.1143/JPSJ.26.993
Longo JM, Kafalas JA (1969) The effect of pressure and B-cation size on the crystal structure of CsBF3 compounds (B=Mn, Fe, Co, Ni, Zn, Mg). J Solid State Chem 1:103–108. https://doi.org/10.1016/0022-4596(69)90015-2
Novikov AN, Kalinov VS, Pavalanski EI et al. (2019) Luminescence and electron-phonon interaction characteristics of radiation-induced point defects in magnesium fluoride nanocrystals. J Lumin 209:244–250. https://doi.org/10.1016/j.jlumin.2019.01.037
Zhang X, Quan Z, Yang J et al. (2008) Solvothermal synthesis of well-dispersed MF 2 (M = Ca,Sr,Ba) nanocrystals and their optical properties. Nanotechnology 19:075603. https://doi.org/10.1088/0957-4484/19/7/075603
Li C, Xu Z, Yang D et al. (2012) Well-dispersed KRE 3 F 10 (RE = Sm–Lu, Y) nanocrystals: solvothermal synthesis and luminescence properties. CrystEngComm 14:670–678. https://doi.org/10.1039/C1CE06087B
Hall TPP, Leggeat A (1969) Defect centres in KMgF3 produced by X-irradiation at room temperature. Solid State Commun 7:1657–1659. https://doi.org/10.1016/0038-1098(69)90050-7
Hall TPP (1966) The structure of the self-trapped hole in KMgF 3. Br J Appl Phys 17:1011–1018. https://doi.org/10.1088/0508-3443/17/8/306
Riley CR, Sibley WA (1970) Color centers. Phys Rev B 1:2789–2798. https://doi.org/10.1063/1.3060115
Duragkar A, Muley A, Pawar NR et al. (2019) Versatility of thermoluminescence materials and radiation dosimetry – a review. Luminescence 34:656–665. https://doi.org/10.1002/bio.3644
Salah N (2011) Nanocrystalline materials for the dosimetry of heavy charged particles: a review. Radiat Phys Chem 80:1–10. https://doi.org/10.1016/j.radphyschem.2010.08.003
Townsend PD, Wang Y, McKeever SWS (2021) Spectral evidence for defect clustering: Relevance to radiation dosimetry materials. Radiat Meas 147:106634. https://doi.org/10.1016/j.radmeas.2021.106634
Kizhaev SA, Markova LA (2011) Structural and magnetic phase transitions in KMnF3. Solid State Phys 53:1851–1854. https://doi.org/10.1134/S1063783411090162
Lee K, Portis AM, Witt GL (1963) Magnetic properties of the hexagonal antiferromagnet CsMnF3. Phys Rev 132:144–163. https://doi.org/10.1103/PhysRev.132.144
Hirakawa K, Hirakawa K, Hashimoto T (1960) Magnetic properties of potassium iron group fluorides KMF 3. J Phys Soc Jpn 15:2063–2068. https://doi.org/10.1143/JPSJ.15.2063
Malakhovskiĭ AV, Morozova TP (2006) Temperature dependence of the fine structure of the C and E absorption bands in RbMnF3 below the Néel temperature. Phys Solid State 48:283–290. https://doi.org/10.1134/S1063783406020156
Jacquier B, Moncorgé R, Di Bartolo B (1979) Magnon absorption and luminescence in CsMnF3. Solid State Commun 31:693–697. https://doi.org/10.1016/0038-1098(79)90767-1
Garcia-Castro AC, Spaldin NA, Romero AH, Bousquet E (2014) Geometric ferroelectricity in fluoroperovskites. Phys Rev B 89:104107. https://doi.org/10.1103/PhysRevB.89.104107
Lee J-W, Oliveira MT, Jang H, Bin et al. (2016) Hydrogen-bond promoted nucleophilic fluorination: concept, mechanism and applications in positron emission tomography. Chem Soc Rev 45:4638–4650. https://doi.org/10.1039/C6CS00286B
Waseda Y, Matsubara E, Shinoda K (2011) X-Ray Diffraction Crystallography. Springer Berlin Heidelberg, Berlin, Heidelberg
Scherrer P (1918) Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr Ges Wiss Gottingen, Math 98–100.
Niwas Singh R, Padma DK (1994) A study of the synthesis of Group I and Group II metal fluorides with pyridinium poly(hydrogen fluoride), PPHF. J Fluor Chem 67:211–214. https://doi.org/10.1016/0022-1139(93)02965-H
De Keukeleere K, De Roo J, Lommens P et al. (2015) Fast and tunable synthesis of ZrO 2 nanocrystals: mechanistic insights into precursor dependence. Inorg Chem 54:3469–3476. https://doi.org/10.1021/acs.inorgchem.5b00046
Sheng J, Tang K, Su D et al. (2009) Shape-selected synthesis, characterization and optical properties of KMnF3 micropolyhedra, microspheres and hollow microspheres. J Fluor Chem 130:742–748. https://doi.org/10.1016/j.jfluchem.2009.05.018
Williams GVM, Schuyt JJ, Madathiparambil AS (2018) The effect of Mn concentration on the luminescence properties of NaMgF3:Mn: Defect/Mn complex photoluminescence, radioluminescence, and optically stimulated luminescence for radiation dose monitoring. Opt Mater 84:763–770. https://doi.org/10.1016/j.optmat.2018.08.023
Riley CR, Yun SI, Sibley WA (1972) Luminescence from color centers in KMgF3. Phys Rev B 5:3285–3292. https://doi.org/10.1103/PhysRevB.5.3285
Pollock CR (1986) Optical properties of laser-active color centers. J Lumin 35:65–78. https://doi.org/10.1016/0022-2313(86)90039-6
Modak P, Modak B (2021) An ab initio perspective on the key vacancy defects of KMgF 3. J Phys Chem C 125:8253–8267. https://doi.org/10.1021/acs.jpcc.0c10835
Zhou Q, Dolgov L, Srivastava AM et al. (2018) Mn2+ and Mn4+ red phosphors: synthesis, luminescence and applications in WLEDs. A review. J Mater Chem C 6:2652–2671. https://doi.org/10.1039/c8tc00251g
Song E, Ye S, Liu T et al. (2015) Tailored near-infrared photoemission in fluoride perovskites through activator aggregation and super-exchange between divalent manganese ions. Adv Sci 2:1500089. https://doi.org/10.1002/advs.201500089
McKeever SWS (2011) Optically stimulated luminescence: a brief overview. Radiat Meas 46:1336–1341. https://doi.org/10.1016/j.radmeas.2011.02.016
Itoh Y (1974) Calculation of ultrasonic attenuation coefficient in RbMnF 3 near and above the Néel Temperature. J Phys Soc Jpn 36:1204–1204. https://doi.org/10.1143/JPSJ.36.1204
Dubrovin RM, Kizhaev SA, Syrnikov PP et al. (2018) Unveiling hidden structural instabilities and magnetodielectric effect in manganese fluoroperovskites. Phys Rev B 98:060403. https://doi.org/10.1103/PhysRevB.98.060403
Agnoli F, Zhou WL, O’Connor CJ (2001) Synthesis of cubic antiferromagnetic KMnF3 nanoparticles using reverse micelles and their self-assembly. Adv Mater 13:1697–1699. 10.1002/1521-4095(200111)13:22<1697::AID-ADMA1697>3.0.CO;2-F.
Acknowledgements
The authors are grateful to ETH Zürich for the financial support. The authors are grateful to Dr. Alla Sologubenko, of the Scientific Centre for Optical and Electron Microscopy (ScopeM) at ETH Zurich and Dr. Elena Tervoort for TEM measurements and helpful discussions regarding the interpretation of the TEM results. Furthermore, the help of Alexander Firlus for carrying out the magnetic measurements and discussing the results is gratefully acknowledged.
Funding
Open access funding provided by Swiss Federal Institute of Technology Zurich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fellner, M., Lauria, A. Caesium manganese fluoride cubic-perovskite nanoparticles – synthesis, luminescence and magnetic properties. J Sol-Gel Sci Technol 107, 259–268 (2023). https://doi.org/10.1007/s10971-023-06069-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06069-y