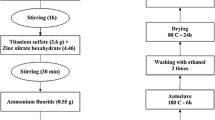
Abstract
Herein, a sol-gel dip-coating scheme has been employed to prepare the high-quality ZnO thin films with various loadings of cobalt (Co) on porous ceramics substrates. The impact of cobalt element on the crystal phase, surface morphology, optical and chemical properties of films were investigated using different spectroscopic techniques including PXRD, FE-SEM, FT-IR,and UV-Vis respectively. The X-rays diffraction pattern showed the synthesis of monophasic ZnO at all Co contents without appearance of any secondary phase; the grain size is decreased from 40 to 12 nm as Co loading scales from 0 to 3%. Scanning electron microscopy study reveals that the existence of Co doping in ZnO impacts its morphology change, and its size is reduced and presented in homogeneous distribution as well as Co content increases, in FT-IR results no secondary phase was observed in the spectra corresponding to Co. In addition, the mean value of reflectance as deduced from UV–Vis spectra lies 86–95% in the visible range, showing Co doping drastically reduces the band gap values from 3.14 to 2.93 eV. The practical applicability was investigated in the removal of Orange II (OII) dye on exposure to ultra violet radiations. In comparison to other samples, the thin films with 5 wt% Co show the highest rate of decomposition in the order of 92% for 4 h irradiation time.
Graphical Abstract

The right side of TOC represents the absorbance spectra of pure ZnO and different cobalt doped ZnO thin films and the left side represents the pure orange II dye and change in the pure orange II dye solutions after 6 h using pure ZnO and different cobalt doped ZnO films catalysts.
Highlights
-
This paper makes an elaborate study on Co-doped ZnO thin films synthesized by sol-gel (dip-coating) method on small porous ceramic support. The different physio-chemical features of the prepared materials are reported here in this issue.
-
The bandgap was calculated by knowing the maximum wavelength from the absorption derivative and applying the following relationship [16], the results show that with the doping concentration, the optical bandgap of the film gradually decreases as indicated in Table 2, this may be due to the Co2+ introduced in the lattice of ZnO.
-
When the dopant concentration (Co) is increased, it is observed that there is an enhancement in the density while the grains size reduces compared to pure ZnO. It is found that when the doping concentration is 5%, the grain size is the most uniform and the film density is best, the grain size is around 20 nm.









Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cui M-H, Cui D, Gao L, Wang A-J, Cheng H-Y (2017) Evaluation of anaerobic sludge volume for improving azo dye decolorization in a hybrid anaerobic reactor with built-in bioelectrochemical system. Chemosphere 169:18–22
Wang W, Huang G, An C, Zhao S, Chen X, Zhang P (2018) Adsorption of anionic azo dyes from aqueous solution on cationic gemini surfactant-modified flax shives: Synchrotron infrared, optimization and modeling studies. J Clean Prod 172:1986–1997
Sun J, Jin J, Beger RD, Cerniglia CE, Chen H (2017) Evaluation of metabolism of azo dyes and their effects on Staphylococcus aureus metabolome. J Ind Microbiol Biotechnol 44:1471–1481
Vaiano V, Sacco O, Libralato G, Lofrano G, Siciliano A, Carraturo F, Guida M, Carotenuto M (2020) Degradation of anionic azo dyes in aqueous solution using a continuous flow photocatalytic packed-bed reactor: Influence of water matrix and toxicity evaluation. J Environ Chem Eng 8:104549
Yu Z, Moussa H, Liu M, Chouchene B, Schneider R, Wang W, Moliere M, Liao H (2018) Tunable morphologies of ZnO films via the solution precursor plasma spray process for improved photocatalytic degradation performance. Appl Surf Sci 455:970–979
Zulfiqar M, Chowdhury S, Samsudin MFR, Siyal AA, Omar AA, Ahmad T, Sufian S (2020) Effect of organic solvents on the growth of TiO2 nanotubes: An insight into photocatalytic degradation and adsorption studies. J Water Process Eng 37:101491
Chen Y, Wang L, Wang W, Cao M (2017) Synthesis of Se-doped ZnO nanoplates with enhanced photoelectrochemical and photocatalytic properties. Mater Chem Phys 199:416–423
Zhu YF, Zhou L, Jiang QS (2020) One-dimensional ZnO nanowires grown on three-dimensional scaffolds for improved photocatalytic activity. Ceram Int 46:1158–1163
Samadipakchin P, Mortaheb HR, Zolfaghari A (2017) ZnO nanotubes: Preparation and photocatalytic performance evaluation. J Photochem Photobiol A: Chem 337:91–99
Aryanto D, Hastuti E, Taspika M, Anam K, Isnaeni I, Widayatno WB, Wismogroho AS, Marwoto P, Nuryadin BW, Noviyanto A (2020) Characteristics and photocatalytic activity of highly c-axis-oriented ZnO thin films. J Sol-Gel Sci Technol 96:226–235
Xu L, Su J, Zheng G, Zhang L (2019) Enhanced photocatalytic performance of porous ZnO thin films by CuO nanoparticles surface modification. Mater Sci Eng: B 248:114405
Zarei S, Hasheminiasari M, Masoudpanah S, Javadpour J (2022) Photocatalytic properties of ZnO/SnO2 nanocomposite films: role of morphology. J Mater Res Technol 17:2305–2312
Al Farsi B, Souier T, Al Marzouqi F, Al Maashani M, Bououdina M, Widatallah H, Al M (2021) Abri, Structural and optical properties of visible active photocatalytic Al doped ZnO nanostructured thin films prepared by dip coating. Optical Mater 113:110868
Islam MR, Azam MG (2020) Enhanced photocatalytic activity of Mg-doped ZnO thin films prepared by sol–gel method. Surf Eng 37:775–783
Khalfallah B, Riahi I, Chaabouni F (2021) Effect of Cu doping on the structural, optical and electrical properties of ZnO thin films grown by RF magnetron sputtering: application to solar photocatalysis. Opt Quant Electron 53:238
Sutanto H, Wibowo S, Arifin M, Hidayanto E (2017) Photocatalytic activity of cobalt-doped zinc oxide thin film prepared using the spray coating technique. Mater Res Express 4:076409
Goswami M (2020) Enhancement of photocatalytic activity of synthesized Cobalt doped Zinc Oxide nanoparticles under visible light irradiation. Opt Mater 109:110400
Roza L, Febrianti Y, Iwan S, Fauzia V (2020) The role of cobalt doping on the photocatalytic activity enhancement of ZnO nanorods under UV light irradiation. Surf Interfac 18:100435
Al-Namshah KS, Shkir M, Ibrahim FA (2021) Hamdy MS, Auto combustion synthesis and characterization of Co doped ZnO nanoparticles with boosted photocatalytic performance. Physica B: Condensed Matter 625:413459
Dhanalakshmi M, Saravanakumar K, Prabavathi SL, Muthuraj V (2020) Iridium doped ZnO nanocomposites: Synergistic effect induced photocatalytic degradation of methylene blue and crystal violet. Inorg Chem Commun 111:107601
P. J, N. Kottam, R. A (2021) Investigation of photocatalytic degradation of crystal violet and its correlation with bandgap in ZnO and ZnO/GO nanohybrid. Inorg Chem Commun 125:108460
Siwińska-Ciesielczyk K, Świgoń D, Rychtowski P, Moszyński D, Zgoła-Grześkowiak A, Jesionowski T (2020) The performance of multicomponent oxide systems based on TiO2, ZrO2 and SiO2 in the photocatalytic degradation of Rhodamine B: mechanism and kinetic studies. Coll Surf A: Physicochem Eng Asp 586:124272
Reddy M, Poojitha P, Rani V, Kumar M (2018) Structural, morphological and photocatalytic properties of Co doped ZnO nanoparticles. J Ovonic Res 14:55–61
Abirami N, Arulanantham A, Wilson KJ (2020) Structural and magnetic properties of cobalt doped ZnO thin films deposited by cost effective nebulizer spray pyrolysis technique. Mater Res Express 7:026405
Khantoul A, Sebais M, Rahal B, Boudine B, Halimi O (2018) Structural and optical properties of ZnO and Co doped ZnO thin films prepared by sol-gel. Acta Phys Pol A 133:114–117
Bindu P, Thomas S (2014) Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J Theor Appl Phys 8:123–134
Vallejo Lozada WA, Cantillo A, Salazar B, Diaz Uribe CE, Ramos Cervantes W, Romero E, Hurtado M (2020) Comparative study of ZnO thin films doped with transition metals (Cu and Co) for methylene blue photodegradation under visible irradiation. Catalysts 10:528
Tseng J-Y, Chen Y-T, Lin CJ, Liu W-J, Wang CC, Chen QR, Cheng MH, Chou TB (2015) The optical, electrical, and nanomechanical properties of ZnO thin films. Optik 126:3263–3266
Yildirim OA, Arslan H, Sönmezoğlu S (2016) Facile synthesis of cobalt-doped zinc oxide thin films for highly efficient visible light photocatalysts. Appl Surf Sci 390:111–121
Acknowledgements
Authors are thankful to Department of Materials Science, Larbi Ben M’hidi University, Oum El Bouaghi, Algeria for funding and supporting the execution of this research. Author contributions KC performed all the experimental work, collected and analyzed the data, and wrote the first draft of the paper. RN and MAP commented on the paper and approved the final version of the paper. AM, AHM, and RN revised the paper and approved the final version of the paper. MAP, KC, FS, and AN conceptualized and designed the work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chehhat, K., Mecif, A., Mahdjoub, A.H. et al. Sol-gel synthesis of porous cobalt-doped ZnO thin films leading to rapid and large scale Orange-II photocatalysis. J Sol-Gel Sci Technol 106, 85–94 (2023). https://doi.org/10.1007/s10971-023-06060-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06060-7