Abstract
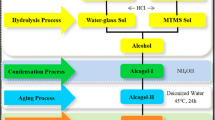
This work presents a facile synthesis approach of methyltriethoxysilane (MTES) based aerogels using pure water as the only solvent, and the effects of precursor concentration on the physicochemical properties are investigated in detail. Therein, the precursor concentration has no effect on the chemical composition but causes the denser and slenderer skeletons at the lower precursor concentration and vice versa. At the 14.4 vol% precursor concentration, the MTES based aerogels are made up of slender skeletons, having the minimum density (0.057 g/cm3), the maximum porosity (97.5%), and the low thermal conductivity (29.4 mW/m/K). It further finds the denser and slenderer silica skeletons cause higher compressive strength and higher Young’s modulus. The TG-DSC results indicate the nice thermal stability of MTES based aerogels. In short, this research demonstrates the great competitive advantages of MTES based aerogels in the field of thermal insulation from the view of preparation method, thermal conductivity, and thermal stability.










Similar content being viewed by others
References
Li Z, Cheng X, Gong L et al. (2018) Enhanced flame retardancy of hydrophobic silica aerogels by using sodium silicate as precursor and phosphoric acid as catalyst. J Non-Cryst Solids 481:267–275. https://doi.org/10.1016/j.jnoncrysol.2017.10.053
Pierre AC, Rigacci A (2011) SiO2 Aerogels. In: Aegerter MA, Leventis N, Koebel MM (eds) Aerogels Handbook. Springer New York, New York, NY, p 21–45
He F, Zhao H, Qu X et al. (2009) Modified aging process for silica aerogel. J Mater Process Technol 209:1621–1626. https://doi.org/10.1016/j.jmatprotec.2008.04.009
Wang Y, Li Z, Huber L et al. (2020) Reducing the thermal hazard of hydrophobic silica aerogels by using dimethyldichlorosilane as modifier. J Sol-Gel Sci Technol 93:111–122. https://doi.org/10.1007/s10971-019-05170-5
Randall JP, Meador MAB, Jana SC (2011) Tailoring mechanical properties of aerogels for aerospace applications. Acs Appl Mater Interfaces 3:613–626. https://doi.org/10.1021/am200007n
Li W, Willey RJ (1997) Stability of hydroxyl and methoxy surface groups on silica aerogels. J Non-Cryst Solids 212:243–249. https://doi.org/10.1016/S0022-3093(97)00021-5
Aegerter MA (2011) Aerogels Handbook. Springer New York, New York, NY
Omranpour H, Dourbash A, Motahari S (2014) Mechanical properties improvement of silica aerogel through aging: Role of solvent type, time and temperature. Nuremberg, Germany 1593:298–302
Mahadik DB, Rao AV, Rao AP et al. (2011) Effect of concentration of trimethylchlorosilane (TMCS) and hexamethyldisilazane (HMDZ) silylating agents on surface free energy of silica aerogels. J Colloid Interface Sci 356:298–302. https://doi.org/10.1016/j.jcis.2010.12.088
Zhang W, Li Z, Shi L et al. (2019) Methyltrichlorosilane modified hydrophobic silica aerogels and their kinetic and thermodynamic behaviors: graphical Abstract. J Sol-Gel Sci Technol 89:448–457. https://doi.org/10.1007/s10971-018-4882-9
Smith D, Deshpande R, Brinke C (1992) Preparation of low-density aerogels at ambient pressure. MRS Proc 271:567–572. https://doi.org/10.1557/PROC-271-567
Soleimani Dorcheh A, Abbasi MH (2008) Silica aerogel; synthesis, properties and characterization. J Mater Process Technol 199:10–26. https://doi.org/10.1016/j.jmatprotec.2007.10.060
Koebel MM, Huber L, Zhao S, Malfait WJ (2016) Breakthroughs in cost-effective, scalable production of superinsulating, ambient-dried silica aerogel and silica-biopolymer hybrid aerogels: from laboratory to pilot scale. J Sol-Gel Sci Technol 79:308–318. https://doi.org/10.1007/s10971-016-4012-5
Huber L, Zhao S, Malfait WJ et al. (2017) Fast and minimal-solvent production of superinsulating silica aerogel granulate. Angew Chem Int Ed 56:4753–4756. https://doi.org/10.1002/anie.201700836
El Rassy H, Buisson P, Bouali B et al. (2003) Surface characterization of silica aerogels with different proportions of hydrophobic groups, dried by the CO2 supercritical method. Langmuir 19:358–363. https://doi.org/10.1021/la020637r
Dong H, Brennan JD (2006) Macroporous monolithic methylsilsesquioxanes prepared by a two-step acid/acid processing method. Chem Mater 18:4176–4182. https://doi.org/10.1021/cm060509e
Rao AV, Kulkarni MM, Amalnerkar DP, Seth T (2003) Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor. J Non-Cryst Solids 330:187–195. https://doi.org/10.1016/j.jnoncrysol.2003.08.048
Xu B, Cai JY, Finn N, Cai Z (2012) An improved method for preparing monolithic aerogels based on methyltrimethoxysilane at ambient pressure Part I: Process development and macrostructures of the aerogels. Microporous Mesoporous Mater 148:145–151. https://doi.org/10.1016/j.micromeso.2011.08.012
Xu B, Cai JY, Xie Z et al. (2012) An improved method for preparing monolithic aerogels based on methyltrimethoxysilane at ambient pressure Part II: Microstructure and performance of the aerogels. Microporous Mesoporous Mater 148:152–158. https://doi.org/10.1016/j.micromeso.2011.08.015
He S, Chen X (2017) Flexible silica aerogel based on methyltrimethoxysilane with improved mechanical property. J Non-Cryst Solids 463:6–11. https://doi.org/10.1016/j.jnoncrysol.2017.02.014
Nadargi DY, Rao AV (2009) Methyltriethoxysilane: New precursor for synthesizing silica aerogels. J Alloy Compd 467:397–404. https://doi.org/10.1016/j.jallcom.2007.12.019
Nadargi DY, Latthe SS, Hirashima H, Rao AV (2009) Studies on rheological properties of methyltriethoxysilane (MTES) based flexible superhydrophobic silica aerogels. Microporous Mesoporous Mater 117:617–626. https://doi.org/10.1016/j.micromeso.2008.08.025
Aravind PR, Soraru GD (2011) High surface area methyltriethoxysilane-derived aerogels by ambient pressure drying. J Porous Mater 18:159–165. https://doi.org/10.1007/s10934-010-9366-4
Niu Z, He X, Huang T et al. (2019) A facile preparation of transparent methyltriethoxysilane based silica xerogel monoliths at ambient pressure drying. Microporous Mesoporous Mater 286:98–104. https://doi.org/10.1016/j.micromeso.2019.05.036
Shao Z, He X, Cheng X, Zhang Y (2017) A simple facile preparation of methyltriethoxysilane based flexible silica aerogel monoliths. Mater Lett 204:93–96. https://doi.org/10.1016/j.matlet.2017.05.104
Michel D (2018) Test of the formal basis of Arrhenius law with heat capacities. Phys Stat Mech Its Appl 510:188–199. https://doi.org/10.1016/j.physa.2018.06.125
Luo Y, Li Z, Zhang W et al. (2019) Rapid synthesis and characterization of ambient pressure dried monolithic silica aerogels in ethanol/water co-solvent system. J Non-Cryst Solids 503–504:214–223. https://doi.org/10.1016/j.jnoncrysol.2018.09.049
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380. https://doi.org/10.1021/ja01145a126
Kumar A, Verma SK, Alvi PA, Jasrotia D (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Yun S, Guo T, Zhang J et al. (2017) Facile synthesis of large-sized monolithic methyltrimethoxysilane-based silica aerogel via ambient pressure drying. J Sol-Gel Sci Technol 83:53–63. https://doi.org/10.1007/s10971-017-4377-0
Rojas F, Kornhauser I, Felipe C et al. (2002) Capillary condensation in heterogeneous mesoporous networks consisting of variable connectivity and pore-size correlation. Phys Chem Chem Phys 4:2346–2355. https://doi.org/10.1039/b108785a
Zhi L, Cheng X, Song H et al. (2016) Aramid fibers reinforced silica aerogel composites with low thermal conductivity and improved mechanical performance. Compos Part Appl Sci Manuf 84:316–325
Li Z, Zhao S, Koebel MM, Malfait WJ (2020) Silica aerogels with tailored chemical functionality. Mater Des 193:108833. https://doi.org/10.1016/j.matdes.2020.108833
Cheng X, Li C, Shi X et al. (2017) Rapid synthesis of ambient pressure dried monolithic silica aerogels using water as the only solvent. Mater Lett 204:157–160. https://doi.org/10.1016/j.matlet.2017.05.107
Lin YF, Hsu SH (2017) Solvent-resistant CTAB-modified polymethylsilsesquioxane aerogels for organic solvent and oil adsorption. J Colloid Interface Ence 485:152–158
Mahadik DB, Rao AV, Parale VG et al. (2011) Effect of surface composition and roughness on the apparent surface free energy of silica aerogel materials. Appl Phys Lett 99:104
Socrates G, Socrates G (2001) Infrared and Raman characteristic group frequencies: tables and charts, 3rd ed. Wiley, Chichester; New York, NY
Al-Oweini R, El-Rassy H (2009) Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R″Si(OR′)3 precursors. J Mol Struct 919:140–145
Li Z, Cheng X, He S et al. (2015) Characteristics of ambient-pressure-dried aerogels synthesized via different surface modification methods. J Sol-Gel Sci Technol 76:138–149. https://doi.org/10.1007/s10971-015-3760-y
Pan Y, He S, Gong L et al. (2017) Low thermal-conductivity and high thermal stable silica aerogel based on MTMS/Water-glass co-precursor prepared by freeze drying. Mater Des 113:246–253. https://doi.org/10.1016/j.matdes.2016.09.083
Wu X, Li Z, Joao G et al. (2020) Reducing the flammability of hydrophobic silica aerogels by tailored heat treatment. J Nanopart Res 22:1–16. https://doi.org/10.1007/s11051-020-04822-w
Huang S, Wu X, Li Z et al. (2020) Rapid synthesis and characterization of monolithic ambient pressure dried MTMS aerogels in pure water. J Porous Mater 27:1241–1251. https://doi.org/10.1007/s10934-020-00902-3
Matias T, Varino C, de Sousa HC et al. (2016) Novel flexible, hybrid aerogels with vinyl- and methyltrimethoxysilane in the underlying silica structure. J Mater Ence 51:6781–6792. https://doi.org/10.1007/s10853-016-9965-9
Li Z, Cheng X, He S (2016) Tailoring thermal properties of ambient pressure dried MTMS/TEOS co-precursor aerogels. Mater Lett 171:91–94. https://doi.org/10.1016/j.matlet.2016.02.025
Lu X, Arduini-Schuster MC, Kuhn J et al. (1992) Thermal conductivity of monolithic organic aerogels. SCIENCE 255:971–2. https://doi.org/10.1126/science.255.5047.971
Koebel M, Rigacci A, Achard P (2012) Aerogel-based thermal superinsulation: an overview. J Sol-Gel Sci Technol 63:315–339. https://doi.org/10.1007/s10971-012-2792-9
Lee OJ, Lee KH, Yim TJ et al. (2002) Determination of mesopore size of aerogels from thermal conductivity measurements. J Non-Cryst Solids 298:287–292. https://doi.org/10.1016/S0022-3093(01)01041-9
Groult S, Budtova T (2018) Thermal conductivity/structure correlations in thermal super-insulating pectin aerogels. Carbohydr Polym 196:73–81. https://doi.org/10.1016/j.carbpol.2018.05.026
Li CH, Jiang SC, Yao ZP et al. (2014) Research on heat transfer characteristics of nano-porous silica aerogel material and its application on mars surface mission. Adv Mater Res 924:329–335. https://doi.org/10.4028/www.scientific.net/AMR.924.329
Li Z, Zhang Y, Huang S et al. (2020) Thermal stability and pyrolysis characteristics of MTMS aerogels prepared in pure water. J Nanopart Res 22:334. https://doi.org/10.1007/s11051-020-05062-8
Huang D, Guo C, Zhang M, Shi L (2017) Characteristics of nanoporous silica aerogel under high temperature from 950 °C to 1200 °C. Mater Des 129:82–90. https://doi.org/10.1016/j.matdes.2017.05.024
Li C, Cheng X, Li Z et al. (2017) Mechanical, thermal and flammability properties of glass fiber film/silica aerogel composites. J Non-Cryst Solids 457:52–59. https://doi.org/10.1016/j.jnoncrysol.2016.11.017
Li Z, Cheng X, Shi L et al. (2016) Flammability and oxidation kinetics of hydrophobic silica aerogels. J Hazard Mater 320:350–358. https://doi.org/10.1016/j.jhazmat.2016.07.054
Rao AV, Kulkarni MM, Amalnerkar DP, Seth T (2003) Surface chemical modification of silica aerogels using various alkyl-alkoxy/chloro silanes. Appl Surf Sci 206:262–270. https://doi.org/10.1016/S0169-4332(02)01232-1
Li Z, Huang S, Shi L et al. (2019) Reducing the flammability of hydrophobic silica aerogels by doping with hydroxides. J Hazard Mater 373:536–546. https://doi.org/10.1016/j.jhazmat.2019.03.112
He S, Huang Y, Chen G et al. (2019) Effect of heat treatment on hydrophobic silica aerogel. J Hazard Mater 362:294–302. https://doi.org/10.1016/j.jhazmat.2018.08.087
He S, Huang D, Bi H et al. (2015) Synthesis and characterization of silica aerogels dried under ambient pressure bed on water glass. J Non-Cryst Solids 410:58–64. https://doi.org/10.1016/j.jnoncrysol.2014.12.011
James MG, Barry JG (1997) Mechanics of Materials, 7 th. Cengage, Canada
Wagh PB, Begag R, Pajonk GM et al. (1999) Comparison of some physical properties of silica aerogel monoliths synthesized by different precursors. Mater Chem Phys 57:214–218. https://doi.org/10.1016/S0254-0584(98)00217-X
Liu GW, Ni XY, Zhou B, Yu QJ (2012) Preparation and characterization of ultralow density silica aerogels by acetonitrile supercritical drying. Key Eng Mater 519:83–86
Gauthier BM, Bakrania SD, Anderson AM, Carroll MK (2004) A fast supercritical extraction technique for aerogel fabrication. J Non-Cryst Solids 350:238–243. https://doi.org/10.1016/j.jnoncrysol.2004.06.044
Zhang Y, Peng et al. (2016) An economic and environmentally benign approach for the preparation of monolithic silica aerogels. RSC Adv 6:1–11. https://doi.org/10.1039/C6RA21050C
Bisson A, Rigacci A, Lecomte D, Achard P (2004) Effective thermal conductivity of divided silica xerogel beds. J Non-Cryst Solids 350:379–384. https://doi.org/10.1016/j.jnoncrysol.2004.08.238
Li Z, Gong L, Li C et al. (2016) Silica aerogel/aramid pulp composites with improved mechanical and thermal properties. J Non-Cryst Solids 454:1–7. https://doi.org/10.1016/j.jnoncrysol.2016.10.015
Lee SE, Ahn YS, Lee JS et al. (2017) Ambient-pressure drying synthesis of high-performance silica aerogel powders by controlling hydrolysis reaction of water glass. J Ceram Process Res 18:777–782
Li Z, Cheng X, Gong L et al. (2017) Enhanced flame retardancy of hydrophobic silica aerogels by using sodium silicate as precursor and phosphoric acid as catalyst. J Non-Cryst Solids 481:267–275. https://doi.org/10.1016/j.jnoncrysol.2017.10.053
Hegde ND, Venkateswara Rao A (2007) Physical properties of methyltrimethoxysilane based elastic silica aerogels prepared by the two-stage sol–gel process. J Mater Sci 42:6965–6971. https://doi.org/10.1007/s10853-006-1409-5
Acknowledgements
The authors deeply appreciate the supports from the National Natural Science Foundation of China (No. 51904336), the Natural Science Foundation of Hunan Province (No. 2020JJ4714), and the Fundamental Research Funds for the Central Universities (No. 202501003 and 202045001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, X., Wu, L., Deng, Y. et al. Effects of precursor concentration on the physicochemical properties of ambient-pressure-dried MTES based aerogels with using pure water as the only solvent. J Sol-Gel Sci Technol 100, 477–488 (2021). https://doi.org/10.1007/s10971-021-05665-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05665-0