Abstract
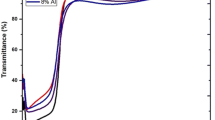
Ice formation on solid surfaces may originate serious problems in various fields such as aircraft, electrical lines etc. Hydrophobic coatings and surfaces can have potential applications with anti-icing properties via excessive repellency of water. Zinc oxide (ZnO) is a material with great importance in many technological applications. Recently, co-doping with different elements in zinc oxide is collecting research interest for hydrophobicity. In this work, calcium co-doped with magnesium in zinc oxide (CaMgZnO) thin films were deposited by sol–gel spin coating method. The doping percentage for calcium was x = 0, 1, 2.5, 4 at% as (CaxMg0.025Zn0.975-x). X-ray diffraction results revealed that all the films had hexagonal wurtzite structure and preferred (002) plane dominance with no secondary phase. Crack free surface was found for all the samples by SEM images. All the deposited films had average transmission of greater than 80%. Maximum band gap value of 3.43 eV revealed for max doping level. Furthermore, optical constants, complex dielectric functions, energy loss functions, and photoluminescence study were also explored. Hydrophobic behavior was evaluated for all the samples and more hydrophobicity with the water contact angle (WCA) of 104.06° produced by the sample having equal amount of calcium and magnesium. De-icing properties like freezing on-set and freezing delay was −15.1 °C and 900 s with the temperature set-point −5 °C respectively. Likewise, maximum freezing delay with temperature set-point −10 °C was 630 s.

Highlights
-
A multifunctional Ca-Mg co-doped ZnO films was prepared by spin-coating method.
-
Surface analysis by SEM showed that the morphology was influenced by doping variations.
-
The nanostructure provides the Ca-Mg co-doped ZnO films with hydrophobicity and ice-phobicity.
-
Optical analysis showed that the transmission of doped ZnO films depends on the doping variation.












Similar content being viewed by others
References
Wang J, Han F, Liang B, Geng G (2017) Hydrothermal fabrication of robustly superhydrophobic cotton fibers for efficient separation of oil/water mixtures and oil-in-water emulsions. J Ind Eng Chem 54:174–183
Wang L, Gong Q, Zhan S, Jiang L, Zheng Y (2016) Adv Mater 28:7729
Kang Z, Li W (2017) Facile and fast fabrication of superhydrophobic surface on magnesium alloy by one-step electrodeposition method. J Ind Eng Chem 50:50–56
Boreyko J, Chen CH (2009) Self-Propelled dropwise condensate on superhydrophobic surfaces. Phys Rev Lett 103:184501
Chu F, Wu X, Zhu B, Zhang X (2016) Self-propelled droplet behavior during condensation on superhydrophobic surface. Appl Phys Lett 108:194103
Li W, Kong C, Qin G, Ruan H, Fang L (2014) p-Type conductivity and stability of AgeN codoped ZnO thin films. J Alloy Compd 609:173–177
Souissi R, Mimouni M, Amlouk S (2015) Guermazi, SiO 2 substrate and Mo, In codoping effect on crystalline and vibrational characteristics of ZnO sprayed thin films. Superlattices Microstruct 85:707–715
Mimouni R, Mahdhi N, Boubaker K, Madouri A, Amlouk M (2016) Physical study on cobalt-indium co-doped ZnO nanofilms as hydrophobic surfaces. Superlattices Microstructures 91:345–357
Fermi Hilbert Inbaraj P, Joseph Prince J (2018) Optical and structural properties of Mg doped ZnO thin film by chemical bath deposition method. J Mater Sci Mater Electron 29:935–943
Srivastava A, Kumar N, Misra KP, Khare S (2014) Blue-light luminescence enhancement and increased band gap from calcium-doped zinc oxide nanoparticle films. Mater Sci Semicond Process 26:259–266
Karthick K, Vijayalakshmi K (2014) Influence of Mg doping on the properties of ZnO films prepared on c-cut sapphire by sputtering. Super Lattice Microstruct 67:172–180
Chen H, Ding J, Ma S (2010) Structural and optical properties of ZnO:Mg thin films grown under different oxygen partial pressure. Phys E 42:1487–1491
Madahi P, Shahtahmasebi N, Kompany A, Mashreghi M, Bagheri-Mohagheghi MM, Hosseini A (2011) Deposition and characterization of ZnO:Mg thin films: the study of antibacterial properties. Phys Scr 84:035801
Vijayalakshmi K, Renitta A, Karthick K (2014) Growth of high quality ZnO:Mg films on ITO coated glass substrates for enhanced H2 sensing. Ceram Int 40:6171–6177
Mia MNH, Pervez MF, Khalid Hossain M, Reefaz Rahman M, Jalaludin M, Almashud MA, Ghosh HK (2017) Mahbubul Haq, Influence of Mg content on tailoring optical bandgap of Mg doped ZnO thin film prepared by sol-gel method. Results Phys 7:2683–2691
Caglar M, Caglar Y, Ilican S (2016) Investigation of the effect of Mg doping for improvement of optical and electrical properties. Phys B 485:6–13
Huang K, Tang Z, Zhang L, Yu J, Lv J, Liu X, Liu F (2012) Preparation and characterization of Mg doped ZnO thin films by sol-gel method. Appl Surf Sci 258:3710–3713
Hussain KA, Aadim HM (2014) Slman, Structural and optical properties of Mg doped ZnO thin films deposited by pulse laser deposition (PLD), Iraqi. J Phys 12:56–61
Verma K, Chaudhary B, Kumar V, Sharma V, Kumar M (2017) Investigation of structural, morphological and optical properties of Mg:ZnO thin films prepared by sol-gel spin coating method. Vacuum 146:524–529
Anca-Ionela I, Florin N, Iuliana M, Florin C, Raluca G, Oana T, Cosmin R, Vasilica T, Nedelcu M, Müller R (2019) Synthesis and characterization of Ca doped ZnO thin films by sol–gel method. J Sol-Gel Sci Technol 92:585–597
Mondal C, Ganguly M, Sinha AK, Pal J, Pal T (2013) Fabrication of a ZnO nanocolumnar thin film on a glass slide and its reversible switching from a superhydrophobic to a superhydrophilic state. RSC Adv 3:5937e5944
Yan B, Tao J, Pang C, Zheng Z, Shen Z, Huan CHA, Yu T (2008) Reversible UV-light-induced ultrahydrophobic-to-ultrahydrophilic transition in an a-Fe2O3 nanoflakes film. Langmuir 24:10569–10571
Sinha AK, Basu M, Pradhan M, Sarkar S, Negishi Y, Pal T (2011) Redox-switchable superhydrophobic silver composite. Langmuir 27:11629–11635
Chaudhary G, Li R (2014) Freezing of water droplets on solid surfaces: an Experimental and Numerical Study. Exp Therm Fluid Sci 57:86–93
Lv J, Song Y, Jiang L, Wang J (2014) Bio-Inspired Strategies for Anti-Icing. ACS Nano 8:3152–3169
Chen J, Liu J, He M, Li K, Cui D, Zhang Q, Zeng X, Zhang Y, Wang J, Song Y (2012) Superhydrophobic surfaces cannot reduce ice adhesion. Appl Phys Lett 101:111603
Graeber G, Schutzius TM, Eghlidi H, Poulikakos D (2017) Spontaneous self-dislodging of freezing water droplets and the role of wettability. Proc Natl Acad Sci 114:11040–11045
Meuler J, McKinley GH, Cohen RE (2010) Exploiting topographical texture to impart icephobicity. ACSNano 4:7048–7052
Laforte JL, Allaire MA, Laflamme J (1998) State-of-the-art on power line de-icing. Atmos Res 46:143–158
Jung S, Dorrestijn M, Raps D, Das A, Megaridis CM, Poulikakos D (2011) A resuper hydrophobic surfaces best for icephobicity? Langmuir 27:3059–3066
Zhang S, Huang J, Cheng Y, Yang H, Chen Z, Lai Y (2017) Bio inspired surfaces with superwettability for anti-icing and ice-phobic application: concept, mechanism, and design. Small 13:1–20.
Jellinek HH (1959) Adhesive properties of ice. J Colloid Sci 14:268–280
Raraty LE, Tabor D (1958) The adhesion and strength properties of ice. Proc R Soc A Math Phys Eng Sci 245:184–201
Schutzius TM, Jung S, Maitra T, Eberle P, Antonini C, Stamatopoulos C, Poulikakos D (2015) Physics of icing and rational design of surfaces with extraordinary icephobicity. Langmuir 31:4807–4821
Lv J, Song Y, Jiang L, Wang J (2014) Bio-inspired strategies for anti-icing. ACSNano 8:3152–3169
Kreder MJ, Alvarenga J, Kim P, Aizenberg J (2016) Design of anti-icing surfaces: smooth, textured or slippery? Nat Rev Mater 1:15003
Amin-Sarshar M, Song D, Swartz CH, Lee J, Choi CH (2018) Anti-icing or deicing: icephobicities of superhydrophobic surfaces with hierarchical structures. Langmuir 34:13821
Fu Q, Wu X, Kumar D, Jeffrey WCHo, Kanhere PD, Srikanth N, Liu E, Wilson P, Chen Z (2014) Development of sol−gel icephobic coatings: effect of surface roughness and surface energy. Appl Mater Interfaces 6:20685–20692
Fu QT, Liu EJ, Wilsonc P, Chen Z (2015) Ice nucleation behaviour on sol–gel coatings with different surface energy and roughness. Phys Chem Chem Phys 17:21492–21500
Eberle P, Tiwari MK, Maitra T, Poulikakos D (2014) Rational nanostructuring of surfaces for extraordinary icephobicity. Nanoscale 6:4874–4881
Wang S, Liu K, Yao X, Jiang L (2015) Bioinspired surfaces with superwettability: new insight on theory, design, and applications. Chem Rev 115:8230–8293
Qi L, Zhiguang G (2018) Fundamentals of icing and common strategies for designing biomimetric anti-icing surfaces. J Mater Chem A 6:13549–13581
Foo KL, Kashif M, Hashim U, Wei-wen L (2014) Effect of different solvents on the structural and optical properties of zinc oxide thin films for optoelectronic applications. Ceram Int 40:753–761
Karzazi O, Soussi L, Louardi A, El Bachiri A, Khaider M, Monkade M, Erguig H, Taleb M (2019) Transparent conducting properties of Mg and Al co-doped ZnO thin films deposited by spray pyrolysis technique. Superlattice Microstruct 127:61–65
Slama R, El Ghoul J, Omri K, Houas A, El Mir L, Launay F (2016) Effect of Ca-doping on microstructure and photocatalytic activity of ZnO nanoparticles synthesized by sol gel method. J Mater Sci Mater Electron 27:7939–7946
Mohamed S, Sayed AMEl (2016) Effects of lanthanum and sodium on the structural, optical and hydrophilic properties of sol-gel derived ZnO films: a comparative study Mater Sci Semiconduct Process 41:323–334
Kumar KDA, Ganesh V, Shkir M, AlFaify S, Valanarasu S (2018) Effect of different solvents on the key structural, optical and electronic properties of sol-gel dip coated AZO nanostructured thin films for optoelectronic applications. J Mater Sci Mater Electron 29:887–897
Bekkari R, Jaber B, Labrim H, Ouafi M, Zayyoun N, Laanab L (2019) Effect of solvents and stabilizer molar ratio on the growth orientation of sol-gel derived ZnO thin films. Hindawi Int J Photoenergy 3164043:7
Kim I, Shin SW, Gang MG, Leea SH, Gurav KV, Patil PS, HoYun J, Lee JY, Kim JH (2014) Comparative study of quaternary Mg and group III element co-doped ZnO thin films with transparent conductive characteristics. Thin Solid Films 570:321–325
Zhuang H, Wang J, Liu H, Li J, Xu P (2011) Structural and optical properties of ZnO nanowires doped with magnesium. Acta Phys Pol A 119:819–823
Baig F, Asif A, Muhammad Waseem A, Muhammad I (2020) Comparative study for seed layer solvent effects on structural and optical properties of MgZnO thin films deposited by chemical bath deposition technique Mater Res Express 7:026417
Shkir M, Arif M, Ganesh V, Manthrammel MA, Singh A, Yahia IS, Maidur SR, Patil PS, AlFaify S (2018) Investigation on structural, linear, nonlinear and optical limiting properties of sol-gel derived nanocrystalline Mg doped ZnO thin film for optoelectronic applications. J Mol Struct 1173:375–384
Lee JH, Ko KH, Park BO (2003) Electrical and optical properties of ZnO transparent conduction film by the sol-gel method. J Cryst Growth 247:119–125
Wakkad MM, Shokr EK, Mohamed SH (2000) Optical and calorimetric studies of Ge-Sb-Se glasses. J Non-Cryst Solids 265:157–166
El-Nahass MM, Soliman HS, Hendi AA, El-Gamdy SH (2011) Aust J Basic Appl Sci 5:145
Kashif M, Usman Ali SM, Ali ME, Abdulgafour HI, Hashim U, Willander M, Hassan Z (2012) Morphological, optical and raman characteristics of ZnO nanoflakes prepared via a sol-gel method, Phys. Status. Solidi A 209:143–147
Lee CT (2010) Fabrication methods and luminescent properties of ZnO materials for light emitting diode. Materials 3:2218–2259
Kashif M, Hashim U, Ali ME, Ali SMU, Rusop M, Ibupoto ZH, Willander M (2012) Effect of different seed solutions on the morphology and electrooptical properties of ZnO nanorods. J Nanomate 452407
Kai LF, Hashim U, Muhammad K, Voon CH (2014) Sol-gel synthesized zinc oxide nanorods and their structural and optical investigation for optoelectronic applications. Nanoscale Res Lett 9:429
Akhtar N, Holm VR, Thomas PJ, Svardal B, Askeland SH, Holst B (2015) Underwater superoleophobic sapphire (0001) surfaces. J Phys Chem C 119:15333–15338
Taherian F, Marcon V, van der Vegt NF, Leroy F (2013) What is the contact angle of water on graphene? Langmuir 29:1457–1465
Akhtar N, Anemone G, Farias D, Holst B (2019) Fluorinated graphene provides long lasting ice inhibition in high humidity. Carbon 141:451–456
Bharathidasan T et al. (2014) Effect of wettability and surface roughness on iceadhesion strength of hydrophilic, hydrophobic and superhydrophobic surfaces. Appl Surf Sci 314:241–250
Alizadeh A et al. (2012) Dynamics of ice nucleation on water repellent surfaces. Langmuir 28:3180–3186
Li K et al. (2012) Investigating the effects of solid surfaces on ice nucleation. Langmuir 28:10751–10753
Boinovich LB et al. (2016) Anti-icing properties of a superhydrophobic surface in a salt environment: an unexpected increase in freezing delay times for weak brine droplets. Phys Chem Chem Phys 18:3131–3136
He M et al. (2010) Super-hydrophobic film retards frost formation. Soft Matter 6:2396
Yang J, Li W (2013) Preparation of superhydrophobic surfaces on Al substrates and the anti-icing behavior. J Alloy Compd 576:215–219
Liu Z et al. (2008) Frost formation on a super-hydrophobic surface under natural convection conditions. Int J Heat Mass Transf 51:5975–5982
Kulinich SA, Farzaneh M (2009) Ice adhesion on super-hydrophobic surfaces. Appl Surf Sci 255:8153–8157
Zhang X et al. (2013) Self-cleaning superhydrophobic surface based on titanium dioxide nanowires combined with polydimethylsiloxane. Appl Surf Sci 284:319–323
Bixler GD et al. (2014) Anti-fouling properties of microstructured surfaces bioinspired by rice leaves and butterfly wings. J Colloid Interface Sci 419:114–133
Chen Y et al. (2012) Transparent superhydrophobic/superhydrophilic coatings for self-cleaning and anti-fogging. Appl Phys Lett 101:033701
Chang KC et al. (2013) Nanocasting technique to prepare lotus-leaf-like superhydrophobic electroactive polyimide as advanced anticorrosive coatings. ACS Appl Mater Interfaces 5:1460–1467
Mishchenko L et al. (2010) Design of ice-free nanostructured surfaces based on repulsion of impacting water droplets. ACS Nano 4:7699–7707
Antonini C et al. (2011) Understanding the effect of superhydrophobic coatings on energy reduction in anti-icing systems. Cold Reg Sci Technol 67:58–67
Acknowledgements
The authors would like thanks to Head of Physics Department and Nano Electronics Research lab, GC University Lahore. FB would like thanks to HEC Pakistan for IRSIP scholarship to conduct research in University of Bergen Norway. He also acknowledges the Head of Nano-Physics group Department of Physics and Technology, University of Bergen Norway.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baig, F., Asif, A., Ashraf, M.W. et al. Tailoring of optical, hydrophobic, and anti-icing properties of Ca–Mg co-doped ZnO thin films via sol–gel method. J Sol-Gel Sci Technol 97, 706–720 (2021). https://doi.org/10.1007/s10971-020-05464-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05464-z