Abstract
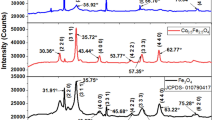
The solid solution NiFe2-xMnxO4 (0 ≤ x ≤ 2) is synthesized by sol gel method and the physical properties are investigated for the first time. The thermal analysis shows that the phases are formed above 400 °C. The oxides crystallize in an inverse cubic spinel whose lattice constant (a: 0.83381–0.83985 nm) increases only slightly up to x = 1.6, according to the Vegard’s law; such result is supported by the FTIR spectra. The UV-Visible spectroscopy shows both direct (1.00–1.56 eV) and indirect optical transitions (0.39–1.65 eV) due to the crystal field splitting of 3d metal. Field-dependent magnetization of the solid solution was measured at 300 K in the region (±20 kOe) and the end member NiFe2O4 exhibits a high magnetism with a saturation magnetization (20 emu/g), comparable to that reported previously. The saturation magnetization varies between 0.5 and 20 emu/g while the coactivity fluctuates between 110 and 239 Oe. The thermal variation of the electrical conductivity indicates a conduction mechanism by low polaron hopping which follows an exponential law with variable activation energies (Ea: 0.12–033 eV). The thermo-power is positive and nearly constant (S300K: 144–130 µVK−1), indicating p type conduction with a mobility more a less constant (1.5–8 × 10−6 V2 cm−1 s−1). The hole densities (NA × 1016: 0.26–2.17) are determined electrochemically from the capacitances measurements in neutral medium (Na2SO4 0.5 M). The flat band potential (Efb: −0.04 to −0.29 V) does not change significantly indicating that the valence band derives mostly from 3d orbital.

Highlights
-
The solid solution NiFe2-xMnxO4 (0 £ × £ 2) is synthesized by sol gel method.
-
NiFe2-xMnxO4 system exhibits p-type conductivity with direct transition band gaps.
-
The charge transport occurs either between Fe2+/Fe3+ or Mn2+/Mn3+ ions in B-sites.









Similar content being viewed by others
References
Habibi MH, Mardani M (2017) Synthesis and characterization of bi-component ZnSnO3/Zn2 SnO4 (perovskite / spinel) nano-composites for photocatalytic degradation of intracron blue: structural, opto-electronic and morphology study. J. Mol. Liq. 238:397–401.
Ford JC, Zakutayev A, Ndione PF, Sigdel AK, Widjonarko NE, Parilla PA et al. (2019) Opto-electronic properties of Co-Zn-Ni-O films deposited by RF-sputtering at ambient-temperature. J. Alloys Compd 801:409–414.
Rekhila G, Brahimi R, Bessekhouad Y, Trari M (2017) Physical and photoelectrochemical characterizations of the pyrochlore La1.9Ba0.1Sn2O7: application to chromate reduction under solar light. J Photochem Photobiol A: Chem 332:345–350.
Mishra M, Zhang Y, Mishra D, Sahoo MPK, Pradhan PK, Pattanaik AK, Defect driven d0 ferromagnetism and colossal dielectric behavior in Bi(Zn0.5Ti0.5)O3–PbTiO3 ceramics, Ceram. Int. https://doi.org/10.1016/j.ceramint.2019.07.338.
Oliveira RGM, Silva RA, De Morais JEV, Batista GS, Silva MAS, Goes JC et al. (2019) Effects of CaTiO3 addition on the microwave dielectric properties and antenna properties of BiVO4 ceramics. Compos. B. Eng. 175:107122.
Haddadou N, Bensemma N, Rekhila G, Trari M, Taïbi K (2018) Chemistry photoelectrochemical investigations in lead-free Ba application to amoxicillin photodegradation. J Photochem Photobiol A 358:294–299.
Zhang Y, Ma C, Yang X, Song Y, Liang X, Zhao X, et al. NASICON-based gas sensor utilizing MMnO3 (M: Gd, Sm, La) sensing electrode for triethylamine detection Sens Actuators B Chem 2019;295:56–64.
Kaizra S, Louafi Y, Bellal B, Trari M, Rekhila G (2015) Electrochemical growth of tin(II) oxide films: application in photocatalytic degradation of methylene blue. Mater Sci Semicond Process 30:554–560.
Rekhila G, Bessekhouad Y, Trari M (2016) Synthesis and characterization of the spinel ZnFe2O4, application to the chromate reduction under visible light. Environ Technol Innov 5:127–135.
Zyoud A, Murtada K, Kwon H, Choi H, Woo T, Helal MHS, et al. (2020) Copper selenide film electrodes prepared by combined electrochemical / chemical bath depositions with high photo-electrochemical conversion efficiency and stability. Solid State Sci 1–10.
Rekhila G, Bessekhouad Y, Trari M (2015) Hydrogen evolution under visible light over the solid solution NiFe2-xMnxO4 prepared by sol gel. Int J Hydrogen Energy 40:12611–12618.
Boutal N, Rekhila G, Taïbi K, Trari M (2014) Relaxor ferroelectric and photo-electrochemical properties of lead-free Ba1-xEu2x/3(Ti0.75Zr0.25)O3 ceramics. Application to chromate reduction. Solar Energy 99:291–298.
Bensemma N, Rekhila G, Boutal N, Taïbi K, Trari M (2016) Photoelectrochemical properties of lead-free ferroelectric ceramic Ba(Ti0.96Mg0.013Nb0.026)O3: application to solar conversion of eosin. J Mater Sci: Mater Electr 27:6757–6765.
Roumila Y, Abdmeziem K, Rekhila G, Trari M (2016) Semiconducting properties of hydrothermally synthesized libethenite application to orange G photodegradation. Mater Sci Semicond Process 41:470–479.
Xavier F, Santos I, Oliveira R, Alves E, Júnior A, Lopes M, et al. Antimicrobial properties of α -Ag2WO4 rod-like microcrystals synthesized by sonochemistry and sonochemistry followed by hydrothermal conventional method. Ultrasonics - Sonochem 2019;58.
Dabagh S, Chaudhary K, Haider Z, Ali J (2018) Study of structural phase transformation and hysteresis behavior of inverse-spinel a -ferrite nanoparticles synthesized by co-precipitation method. Solid State Sci 8:93–98.
Bui TQ, Ton SN, Duong AT, Tran HT (2018) Size-dependent magnetic responsiveness of magnetite nanoparticles synthesised by co-precipitation and solvothermal methods. J Sci Adv Mater Dev 3:107–112.
Rekhila G, Gabes Y, Bessekhouad Y, Trari M (2018) Hydrogen production under visible illumination on the spinel NiMn2O4prepared by sol gel. Solar Energy 166:220–225.
Haruna A, Abdulkadir I, Idris SO (2020) Science Synthesis, characterization and photocatalytic properties of nanoparticles using the sol-gel method. J King Saud Univ Sci. 32:896–903.
Rekhila G, Gabes Y, Brahimi R, Bessekhouad Y, Mahroua O, Trari M (2018) Preparation and characterization of the system NiMn2O4/TiO2 by sol–gel: application to the photodegradation of benzamide under visible light. J Sol-Gel Sci Technol 85:677–683.
Liang J, Wang J, Song K, Wang X, Yu K, Liang C, Enhanced photocatalytic activities of Nd-doped TiO2 under visible light using a facile sol-gel method. J Rare Earth. https://doi.org/10.1016/j.jre.2019.07.008.
Rekhila G, Bessekhouad Y, Trari M (2013) Visible light hydrogen production on the novel ferrite NiFe2O4. Int J Hydrog Energy 8:0–8.
Abid H, Rekhila G, Ihaddadene FA, Bessekhouad Y, Trari M (2019) Hydrogen evolution under visible light illumination on the solid solution Cd x Zn 1-x S prepared by ultrasound-assisted route. Int J Hydrog Energy 44:0301–10308.
Shannon RD Revised effective ionic radii and systematic study of inter atomic distances in halides and chalcogenides in halides and chaleogenides 2016. https://doi.org/10.1107/s0567739476001551.
Cherifi K, Rekhila G, Omeiri S, Bessekhouad Y, Trari M (2019) Chemistry Physical and photoelectrochemical properties of the spinel ZnCr2O4 prepared by sol gel: application to Orange II degradation under solar light. J Photochem Photobiol A 368:290–295.
Gherbi R, Bessekhouad Y, Trari M Optical and transport properties of Sn-doped ZnMn2O4 prepared by sol – gel method 2016 J Phys Chem Solids; 89:69–77.
Singh RN, Singh JP, Lal B, Singh A for electrocatalysis of oxygen evolution in alkaline solutions 2007;32:11–16.
Jun S, Hun S, Hong K, Soo K Electrical and magnetic properties of spinel ZnCr2−xFexO4 (0 ≤ x ≤ 1.0) 2002;73:330–334.
Bhagwan J, Rani S, Sivasankaran V, Yadav KL, Sharma Y Improved energy storage, magnetic and electrical properties of aligned, mesoporous and high aspect ratio nanofibers of 2017 Appl Surf Sci;426:913–923.
He Z, Xia Y, Su J, Tang B (2019) Fabrication of magnetically separable NiFe2O4/Bi24O31Br10 nanocomposites and excellent photocatalytic performance under visible light irradiation. Opt. Mater. 88:195–203.
Boukhemikhem Z, Brahimi R, Rekhila G, Fortas G, Boudjellal L, Trari M The photocatalytic hydrogen formation and NO2− oxidation on the hetero-junction Ag/NiFe2O4 prepared by chemical route. Renew Energy 2020;145. https://doi.org/10.1016/j.renene.2019.08.021.
Helaïli N, Bessekhouad Y, Bachari K, Trari M Synthesis and physical properties of the CuFe2-xMnxO4 solid solution 2014;148:734–743.
Boukhemikhem Z, Rekhila G, Brahimi R, Trari M (2020) Chemistry photocatalytic NO2− oxidation on the hetero-junction Ag (5 %)/ NiFe2O4 prepared by sol gel route. J Photochem Photobiol A: Chem 394:112454.
Rekhila G, Trari M, Bessekhouad Y (2017) Characterization and application of the hetero-junction ZnFe2O4/TiO2 for Cr(VI) reduction under visible light. Appl Water Sci 7:1273–1281.
Meziani D, Reziga A, Rekhila G, Bellal B, Trari M Hydrogen evolution under visible light over LaCoO3 prepared by chemical route. Energy Convers Manag 2014;82. https://doi.org/10.1016/j.enconman.2014.03.028.
Cherifi K, Allalou N, Rekhila G, Trari M, Bessekhouad Y Nitrate-processing and characterization of a cobalt-doped barium tin oxide perovskite: magnetic, transport and photoelectrochemical properties. Mater Sci Semicond Process 2015;30. https://doi.org/10.1016/j.mssp.2014.10.018.
Acknowledgements
We acknowledge the financial support from the Thematic Research Agency for Science and Technology (ATRST) of Algeria, through the national research program (PM 01/2019, CNEPRU Project N° B002014N160420190001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rekhila, G., Cherifi, K., Bessekhouad, Y. et al. Physical properties of the solid solution NiFe2-xMnxO4 prepared by sol gel. J Sol-Gel Sci Technol 97, 540–547 (2021). https://doi.org/10.1007/s10971-020-05456-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05456-z