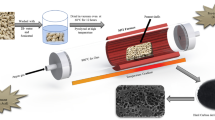
Abstract
Water storage and purification, as well as dehydration processes, are a cause of concern for many healthy and industrial issues. In this work, calcium phosphate (CaP) materials with high water capture performance were prepared by sol–gel synthesis. After their structural characterization by several techniques, they were used in water capture tests performed in temperatures ranging from 25 to 45 °C using a climatic chamber kept at a relative humidity of 60%. The CaP samples showed a higher water uptake capacity than a commercial silica sample commonly used as a water adsorbent, despite the low surface area and pore volume displayed by the former. Moreover, different from the silica adsorbent, the studied CaP materials were submerged by the captured water after the first day, which suggests a deliquescent behavior. The CaP mass uptakes reached around 350% when tested at 25 °C and the materials release the adsorbed water quickly when heated at 100 °C. It was also demonstrated that the materials prepared here maintained their structural integrity even after the deliquescence step, which could allow their use for many cycles.

Highlights
-
Calcium phosphate materials were prepared by sol–gel synthesis.
-
Materials consist of CaHPO4 crystals with an amorphous phase.
-
Calcium phosphate (CaP) samples present high water capture capacities.
-
Sample structures do not contain a significant volume of mesopores.
-
Synthesized CaP materials present deliquescent behaviors.








Similar content being viewed by others
References
Farrusseng D (2011) Metal-organic frameworks: applications from catalysis to gas storage. Wiley-VCH, Weinheim
Canivet J, Fateeva A, Guo Y et al. (2014) Water adsorption in MOFs: fundamentals and applications. Chem Soc Rev 43:5594–5617. https://doi.org/10.1039/c4cs00078a
Furukawa H, Gándara F, Zhang Y-B et al. (2014) Water adsorption in porous metal-organic frameworks and related materials. J Am Chem Soc 136:4369–81. https://doi.org/10.1021/ja500330a
Becker PS (1972) Deliquescent desiccant gas dryer and method. US Patent 3,653,181
Yao W, Yu X, Lee JW et al. (2011) Measuring the deliquescence point of crystalline sucrose as a function of temperature using a new automatic isotherm generator. Int J Food Prop 14:882–893. https://doi.org/10.1080/10942910903474393
Tereshchenko AG (2015) Deliquescence: hygroscopicity of water-soluble crystalline solids. J Pharm Sci 104:3639–3652. https://doi.org/10.1002/jps.24589
Thiel PA, Madey TE (1987) The interaction of water with solid surfaces: fundamental aspects. Surf Sci Rep 7:211–385
Salameh AK, Mauer LJ, Taylor LS (2006) Deliquescence lowering in food ingredient mixtures. J Food Sci 71:E10–E16. https://doi.org/10.1111/j.1365-2621.2006.tb12392.x
Reports SS, Henderson M, Northwest P (2016) The interaction of water with solid surfaces: fundamental aspects revisited. Surf Sci Rep 46:1–308. https://doi.org/10.1016/S0167-5729(01)00020-6
Mauer LJ, Taylor LS (2010) Water-solids interactions: deliquescence. Annu Rev Food Sci Technol 1:41–63. https://doi.org/10.1146/annurev.food.080708.100915
van Campen L, Amidon GL, Zografi G (1983) Moisture sorption kinetics for water-soluble substances. I: theoretical considerations of heat transport control. J Pharm Sci 72:1381–1388. https://doi.org/10.1002/jps.2600721204
Marques CF, Olhero S, Abrantes JCC et al. (2017) Biocompatibility and antimicrobial activity of biphasic calcium phosphate powders doped with metal ions for regenerative medicine. Ceram Int 43:15719–15728. https://doi.org/10.1016/j.ceramint.2017.08.133
Dawood AE, Manton DJ, Parashos P et al. (2018) Biocompatibility and osteogenic/calcification potential of casein phosphopeptide-amorphous calcium phosphate fluoride. J Endod 44:452–457. https://doi.org/10.1016/j.joen.2017.11.005
Zhao M, Dai Y, Li X et al. (2018) Evaluation of long-term biocompatibility and osteogenic differentiation of graphene nanosheet doped calcium phosphate-chitosan AZ91D composites. Mater Sci Eng C 90:365–378. https://doi.org/10.1016/j.msec.2018.04.082
Chen X, Wright JV, Conca JL, Peurrung LM (1997) Evaluation of heavy metal remediation using mineral apatite. Water Air Soil Pollut 98:57–78. https://doi.org/10.1023/A:1026425931811
Ozawa M, Satake K, Suzuki S (2003) Removal of aqueous manganese using fish bone hydroxyapatite. J Mater Sci Lett 22:1363–1364. https://doi.org/10.1023/A:1025795529901
Plec I, Dimovic S, Mitric M (2006) Removal of Co2+ from aqueous solutions by hydroxyapatite. Water Res 40:2267–2274. https://doi.org/10.1016/j.watres.2006.04.031
Xu H, Yang L, Wang P, Yu L, Peng M (2007) Removal mechanism of aqueous lead by a novel eco-material: carbonate hydroxyapatite. J Mater Sci Technol 23:417
Sanosh KP, Chu MC, Balakrishnan A et al. (2010) Sol-gel synthesis of pure nano sized β-tricalcium phosphate crystalline powders. Curr Appl Phys 10:68–71. https://doi.org/10.1016/j.cap.2009.04.014
Wang J, Shaw LL (2009) Synthesis of high purity hydroxyapatite nanopowder via sol–gel combustion process. J Mater Sci Mater Med 20:1223–1227. https://doi.org/10.1007/s10856-008-3685-x
Fernández-pacheco R, Marquina C, Gupta R, Kumar A (2008) Bioactive materials for biomedical applications using sol–gel technology. Biomed Mater 3:034005. https://doi.org/10.1088/1748-6041/3/3/034005
Houmard M, Fu Q, Saiz E, Tomsia AP (2012) Sol-gel method to fabricate CaP scaffolds by robocasting for tissue engineering. J Mater Sci Mater Med 23:921–930. https://doi.org/10.1007/s10856-012-4561-2
Rodrigues Mota TL, Marques de Oliveira AP, Nunes EHM, Houmard M (2017) Simple process for preparing mesoporous sol-gel silica adsorbents with high water adsorption capacities. Microporous Mesoporous Mater 253:177–182. https://doi.org/10.1016/j.micromeso.2017.07.010
Raynaud S, Champion E, Bernache-Assollant D, Thomas P (2002) Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 23:1065–1072. https://doi.org/10.1016/S0142-9612(01)00218-6
Varma HK, Babu SS (2005) Synthesis of calcium phosphate bioceramics by citrate gel pyrolysis method. Ceram Int 31:109–114. https://doi.org/10.1016/j.ceramint.2004.03.041
Mobasherpour I, Heshajin MS, Kazemzadeh A, Zakeri M (2007) Synthesis of nanocrystalline hydroxyapatite by using precipitation method. J Alloy Compd 430:330–333. https://doi.org/10.1016/j.jallcom.2006.05.018
Bekiaris G, Peltre C, Jensen LS, Bruun S (2016) Using FTIR-photoacoustic spectroscopy for phosphorus speciation analysis of biochars. Spectrochim Acta Part A 168:29–36. https://doi.org/10.1016/j.saa.2016.05.049
ElBatal HA, Khalil EMA, Hamdy YM (2009) In vitro behavior of bioactive phosphate glass-ceramics from the system P2O5-Na2O-CaO containing titania. Ceram Int 35:1195–1204. https://doi.org/10.1016/j.ceramint.2008.06.004
Meejoo S, Maneeprakorn W, Winotai P (2006) Phase and thermal stability of nanocrystalline hydroxyapatite prepared via microwave heating. Thermochim Acta 447:115–120. https://doi.org/10.1016/j.tca.2006.04.013
Fathi MH, Hanifi A, Mortazavi V (2008) Preparation and bioactivity evaluation of bone-like hydroxyapatite nanopowder. J Mater Process Technol 202:536–542. https://doi.org/10.1016/j.jmatprotec.2007.10.004
Chukanov NV, Chervonnyi AD (2016) Some general aspects of the application of IR spectroscopy to the investigation of minerals. In: Infrared spectroscopy of minerals and related compounds. Springer, Cham, p 1–49
Victoria EC, Gnanam FD (2002) Synthesis and characterisation of biphasic calcium phosphate. Trends Biomater Artif Organs 16:12–14
Brockner W, Ehrhardt C, Gjikaj M (2007) Thermal decomposition of nickel nitrate hexahydrate, Ni(NO3)2·6H2O, in comparison to Co(NO3)2·6H2O and Ca(NO3)2·4H2O. Thermochim Acta 456:64–68. https://doi.org/10.1016/j.tca.2007.01.031
Anjaneyulu U, Pattanayak DK, Vijayalakshmi U (2016) Snail shell derived natural hydroxyapatite: Effects on NIH-3T3 cells for orthopedic applications. Mater Manuf Process 31:206–216. https://doi.org/10.1080/10426914.2015.1070415
Takahashi K, Hayakawa T, Yoshinari M et al. (2005) Molecular precursor method for thin calcium phosphate coating on titanium. Thin Solid Films 484:1–9. https://doi.org/10.1016/j.tsf.2004.12.052
Sing KSW (1982) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 54:2201–2218. https://doi.org/10.1351/pac198557040603
Thommes M, Kaneko K, Neimark AV et al. (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069. https://doi.org/10.1515/pac-2014-1117
Thomson GW (1946) The antoine equation for vapor-pressure data. Chem Rev 38:1–39. https://doi.org/10.1021/cr60119a001
Taylor RM, Kuennen RW (1994) Removing lead in drinking water with activated carbon. Environ Prog 13:65–71. https://doi.org/10.1002/ep.670130124
Starov VM (2010) Surface forces action in a vicinity of three phase contact line and other current problems in kinetics of wetting and spreading. Adv Colloid Interface Sci 161:139–152
Jensen KR, Fojan P, Jensen RL, Gurevich L (2014) Water condensation: a multiscale phenomenon. J Nanosci Nanotechnol 14:1859–1871
Lagergren S (1898) Zur Theorie der Sogenannten Adsorption Gelöster Stoffe. K Sven Vetenskapsakad Handl 24:1–39
Yuh-Shan H (2004) Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59:171–177. https://doi.org/10.1023/B:SCIE.0000013305.99473.cf
Oliveira LS, Franca AS, Alves TM, Rocha SDF (2008) Evaluation of untreated coffee husks as potential biosorbents for treatment of dye contaminated waters. J Hazard Mater 155:507–512. https://doi.org/10.1016/j.jhazmat.2007.11.093
Ho Y, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Dördelmann G, Kozlova D, Karczewski S et al. (2014) Calcium phosphate increases the encapsulation efficiency of hydrophilic drugs (proteins, nucleic acids) into poly(d,l-lactide-co-glycolide acid) nanoparticles for intracellular delivery. J Mater Chem B 2:7250–7259. https://doi.org/10.1039/c4tb00922c
Petta D, Fussell G, Hughes L et al. (2016) Calcium phosphate/thermoresponsive hyaluronan hydrogel composite delivering hydrophilic and hydrophobic drugs. J Orthop Transl 5:57–68. https://doi.org/10.1016/j.jot.2015.11.001
Park JW, Jang JH, Lee CS, Hanawa T (2009) Osteoconductivity of hydrophilic microstructured titanium implants with phosphate ion chemistry. Acta Biomater 5:2311–2321. https://doi.org/10.1016/j.actbio.2009.02.026
Acknowledgements
The authors thank the financial support from CNPq (305013/2017-3 and 301423/2018-0), CAPES (PROEX), and FAPEMIG (APQ-01881-18). The UFMG Microscopy Center is acknowledged for the support given in SEM. We also thank Samuel Lima, Ilda Batista, Luiz Oliveira, and Victor Araújo for the support provided in XRD, N2 adsorption, FTIR, and DSC.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fonseca, R.L.M., de Souza Gonçalves, B., Balarini, J.C. et al. Deliquescent behavior of calcium phosphate materials synthesized by sol–gel technique. J Sol-Gel Sci Technol 97, 404–413 (2021). https://doi.org/10.1007/s10971-020-05425-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05425-6