Abstract
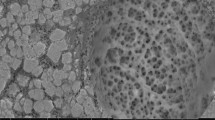
Polymeric aerogels, with their versatile physicochemical properties and capacity for functionalization, are innovative materials being increasingly explored for water treatment applications. In this study, novel millimetric sized alginate-based aerogel granules functionalized with nZVI (nanoscale zero-valent iron) were developed and evaluated for their phosphate sequestration performance. Efficient phosphate removal from water is critical as excessive levels of phosphates can lead to eutrophication and negatively impact water quality. nZVI-aerogel granules exhibited significant enhancements in phosphate removal efficiencies (up to 97%) compared to non-functionalized bare-aerogel granules (15%). Average Langmuir removal capacities of 77 mg-PO43−/g were observed consistently for nZVI-aerogel granules across a broad pH range from 3 to 7, which further increased under alkaline conditions reaching up to 180 mg-PO43−/g at pH 11. Kinetic studies were well described by the pseudo first-order kinetic model in the pH 3–7 range, with rates declining from 0.11 h−1 to 0.07 h−1 as pH increased. In contrast, mixed kinetic trends were observed in alkaline pH with rapid phosphate removal followed by a short-term desorption. Solution pH measurements, and analysis of nZVI-aerogel granule surface chemistry and morphology post batch experiments revealed the involvement of multiple sequestration mechanisms including electrostatic adsorption, ion exchange, and surface precipitation. nZVI-aerogel granule morphology remained stable under all tested conditions (except at pH 11) suggesting their strong potential for facilitating efficient post-treatment separation and recovery.







Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C (2009) G.E. Likens, Controlling eutrophication: nitrogen and phosphorus. American Association for the Advancement of Science
Holt M (2000) Sources of chemical contaminants and routes into the freshwater environment. Food Chem Toxicol 38:S21–S27
Wilfert P, Kumar PS, Korving L, Witkamp G-J, van Loosdrecht MC (2015) The relevance of phosphorus and iron chemistry to the recovery of phosphorus from wastewater: a review. Environ Sci Technol 49(16):9400–9414
Mulkerrins D, Dobson A, Colleran E (2004) Parameters affecting biological phosphate removal from wastewaters. Environ Int 30(2):249–259
Mitrogiannis D, Psychoyou M, Baziotis I, Inglezakis VJ, Koukouzas N, Tsoukalas N, Palles D, Kamitsos E, Oikonomou G, Markou G (2017) Removal of phosphate from aqueous solutions by adsorption onto ca (OH) 2 treated natural clinoptilolite. Chem Eng J 320:510–522
Karageorgiou K, Paschalis M, Anastassakis GN (2007) Removal of phosphate species from solution by adsorption onto calcite used as natural adsorbent. J Hazard Mater 139(3):447–452
Paul E, Laval M, Sperandio M (2001) Excess sludge production and costs due to phosphorus removal. Environ Technol 22(11):1363–1371
Bhargava D, Sheldarkar S (1993) Use of TNSAC in phosphate adsorption studies and relationships. Literature, experimental methodology, justification and effects of process variables. Water Res 27(2):303–312
Hongshao Z, Stanforth R (2001) Competitive adsorption of phosphate and arsenate on goethite. Environ Sci Technol 35(24):4753–4757
Zeng L, Li X, Liu J (2004) Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings. Water Res 38(5):1318–1326
Shanableh AM, Elsergany MM (2013) Removal of phosphate from water using six Al-, Fe-, and Al-Fe-modified bentonite adsorbents. J Environ Sci Health part A 48(2):223–231
Ganesamoorthy R, Vadivel VK, Kumar R, Kushwaha OS, Mamane H (2021) Aerogels for water treatment: a review. J Clean Prod 329:129713
Zhao G, Shi L, Yang G, Zhuang X, Cheng B (2023) 3D fibrous aerogels from 1D polymer nanofibers for energy and environmental applications. J Mater Chem A
García-González CA, Sosnik A, Kalmár J, De Marco I, Erkey C, Concheiro A (2021) Alvarez-Lorenzo, aerogels in drug delivery: from design to application. J Controlled Release 332:40–63
Zhao S, Siqueira G, Drdova S, Norris D, Ubert C, Bonnin A, Galmarini S, Ganobjak M, Pan Z, Brunner S (2020) Additive manufacturing of silica aerogels. Nature 584(7821):387–392
Shan S, Tang H, Zhao Y, Wang W, Cui F (2019) Highly porous zirconium-crosslinked graphene oxide/alginate aerogel beads for enhanced phosphate removal. Chem Eng J 359:779–789
Wang Z, Wu S, Zhang Y, Miao L, Zhang Y, Wu A (2020) Preparation of modified sodium alginate aerogel and its application in removing lead and cadmium ions in wastewater. Int J Biol Macromol 157:687–694
Lentz L, Mayer DA, Dogenski M, Ferreira SRS (2022) Hybrid aerogels of sodium alginate/graphene oxide as efficient adsorbents for wastewater treatment. Mater Chem Phys 283:125981
Zeng H, Sun S, Xu K, Zhao W, Hao R, Zhang J, Li D (2022) Iron-loaded magnetic alginate-chitosan double-gel interpenetrated porous beads for phosphate removal from water: preparation, adsorption behavior and pH stability. Reactive Funct Polym 177:105328
Mukherjee R, Kumar R, Sinha A, Lama Y, Saha AK (2016) A review on synthesis, characterization, and applications of nano zero valent iron (nZVI) for environmental remediation. Crit Rev Environ Sci Technol 46(5):443–466
Tan KB, Vakili M, Horri BA, Poh PE, Abdullah AZ, Salamatinia B (2015) Adsorption of dyes by nanomaterials: recent developments and adsorption mechanisms. Sep Purif Technol 150:229–242
Kumar TP, Mandlimath TR, Sangeetha P, Revathi S, Kumar SA (2018) Nanoscale materials as sorbents for nitrate and phosphate removal from water. Environ Chem Lett 16(2):389–400
Wen Z, Zhang Y, Dai C (2014) Removal of phosphate from aqueous solution using nanoscale zerovalent iron (nZVI). Colloids Surf a 457:433–440
Bhattacharjee S, Darwish N, Shanableh A (2020) Phosphate removal using nanoscale zerovalent iron: impact of chitosan and humic acid. J Environ Chem Eng 8(5):104131
Ayyaril SS, Shanableh A, Bhattacharjee S, Rawas-Qalaji M, Cagliani R, Shabib AG (2023) Recent progress in micro and nano-encapsulation techniques for environmental applications: a review. Results Eng 101094
Eaton AD, Franson MAH, Clesceri LS, Rice EW, Greenberg AE Standard methods for the examination of water & wastewater, Standard methods for the examination of water & wastewater2005, pp. 1. v-1. v
Khalil AM, Eljamal O, Amen TW, Sugihara Y, Matsunaga N (2017) Optimized nano-scale zero-valent iron supported on treated activated carbon for enhanced nitrate and phosphate removal from water. Chem Eng J 309:349–365
Zou F, Budtova T (2021) Tailoring the morphology and properties of starch aerogels and cryogels via starch source and process parameter. Carbohydr Polym 255:117344
Benli B (2013) Effect of borax addition on the structural modification of bentonite in biodegradable alginate-based biocomposites. J Appl Polym Sci 128(6):4172–4180
Pereira CAA, Nava MR, Walter JB, Scherer CE, Dalfovo ADK, Barreto-Rodrigues M (2021) Application of zero valent iron (ZVI) immobilized in Ca-Alginate beads for CI reactive red 195 catalytic degradation in an air lift reactor operated with ozone. J Hazard Mater 401:123275
Kim W, Suh C-Y, Cho S-W, Roh K-M, Kwon H, Song K, Shon I-J (2012) A new method for the identification and quantification of magnetite–maghemite mixture using conventional X-ray diffraction technique. Talanta 94:348–352
Castro RI, Morales-Quintana L, Alvarado N, Guzmán L, Forero-Doria O, Valenzuela-Riffo F, Laurie VF (2021) Design and optimization of a self-assembling complex based on microencapsulated calcium alginate and glutathione (CAG) using response surface methodology, Polymers 13(13) 2080
Yang N, Wang R, Rao P, Yan L, Zhang W, Wang J, Chai F (2019) The fabrication of calcium alginate beads as a green sorbent for selective recovery of Cu (II) from metal mixtures. Crystals 9(5):255
Tang L, Wu P, Zhuang H, Qin Z, Yu P, Fu K, Qiu P, Liu Y, Zhou Y (2023) Nitric oxide releasing polyvinyl alcohol and sodium alginate hydrogels as antibacterial and conductive strain sensors. Int J Biol Macromol 124564
Wai S, Yeap S, Jawad Z (2020) Synthesis of magnetite macro-bead for water remediation: process optimization via manipulation of bead size and surface morphology, IOP Conference Series: Earth and Environmental Science, IOP Publishing, p. 012177
Vetrano A, Gabriele F, Germani R, Spreti N (2022) Characterization of lipase from Candida rugosa entrapped in alginate beads to enhance its thermal stability and recyclability. New J Chem 46(21):10037–10047
Leal D, Matsuhiro B, Rossi M, Caruso F (2008) FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr Res 343(2):308–316
Dehgani Z, Ghaedi M, Sabzehmeidani MM, Adhami E (2020) Removal of paraquat from aqueous solutions by a bentonite modified zero-valent iron adsorbent. New J Chem 44(31):13368–13376
Kumar IA, Viswanathan N (2019) Micro-encapsulation and hydrothermal tuning of amine decorated magnetic alginate hybrid beads for nitrate and phosphate remediation. J Taiwan Inst Chem Eng 102:283–296
Dai M, Zhang Y, Zhang L, Tian Y, Liu G, Zhao J, Liu Y, Zhu S, Ye Z (2022) Multipurpose polysaccharide-based composite hydrogel with magnetic and thermoresponsive properties for phosphorus and enhanced copper (II) removal, composites Part. Appl Sci Manuf 157:106916
Mahmoud AS, Mostafa MK, Nasr M (2019) Regression model, artificial intelligence, and cost estimation for phosphate adsorption using encapsulated nanoscale zero-valent iron. Sep Sci Technol 54(1):13–26
Feng L, Zhang Q, Ji F, Jiang L, Liu C, Shen Q, Liu Q (2022) Phosphate removal performances of layered double hydroxides (LDH) embedded polyvinyl alcohol/lanthanum alginate hydrogels. Chem Eng J 430:132754
Liu A, Liu J, Pan B, Zhang W-x (2014) Formation of lepidocrocite (γ-FeOOH) from oxidation of nanoscale zero-valent iron (nZVI) in oxygenated water. RSC Adv 4(101):57377–57382
Liu A, Liu J, Han J, Zhang W-x (2017) Evolution of nanoscale zero-valent iron (nZVI) in water: microscopic and spectroscopic evidence on the formation of nano-and micro-structured iron oxides. J Hazard Mater 322:129–135
Dall’Agnol P, Junior NL, Muller JM, Xavier JA, Domingos DG, da Costa RHR (2020) A comparative study of phosphorus removal using biopolymer from aerobic granular sludge: a factorial experimental evaluation. J Environ Chem Eng 8(2):103541
Nagoya S, Nakamichi S, Kawase Y (2019) Mechanisms of phosphate removal from aqueous solution by zero-valent iron: a novel kinetic model for electrostatic adsorption, surface complexation and precipitation of phosphate under oxic conditions. Sep Purif Technol 218:120–129
Cheng Q, Li Q, Huang X, Li X, Wang Y, Liu W, Lin Z (2022) The high efficient sb (III) removal by cauliflower like amorphous nanoscale zero-valent iron (A-nZVI). J Hazard Mater 436:129056
Tan AX, Michalski E, Ilavsky J, Jun Y-S (2021) Engineering calcium-bearing mineral/hydrogel composites for effective phosphate recovery. ACS ES&T Eng 1(11):1553–1564
Acknowledgements
We thank the Center of Advanced Materials Research at UoS for assistance with characterization of nanoparticles.
Funding
This work was funded by the University of Sharjah (UoS) grant number UoS-130508. This research work was also partially supported by Abu Dhabi National Oil Company (ADNOC), Emirates NBD and Sharjah Electricity Water & Gas Authority (SEWA), Dubai Electricity and Water Authority R&D Center as the sponsors of the 3rd Forum for Women in Research (QUWA): Women Empowerment for Global Impact at University of Sharjah.
Author information
Authors and Affiliations
Contributions
Sourjya Bhattacharjee (S.B.): Conceptualization, Methodology, Investigation, Formal Analysis, Writing - original draft, Writing - review & editing, Supervision; Abdallah Shanableh (A.S.): Conceptualization, Methodology, Investigation, Formal Analysis, Writing - original draft, Writing - review & editing, Supervision, Funding Acquisition; Sefeera Sadik (S.S.): Investigation, Methodology, Formal Analysis, Writing - original draft.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhattacharjee, S., Shanableh, A. & Sadik, S. Enhanced Phosphate Sequestration by Alginate-based Aerogel Granules Functionalized with Nanoscale Zerovalent Iron. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03318-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03318-1